Looking at hemp as a phytoremediation tool for removing metals from soil
The Cannabis sativa plant has long been recognized for its potential role in removing contaminants from soil in a process known as phytoremediation. However, the use of constructed wetlands and biomass plants as phytoremediants are not new. So how does hemp stack up against what we already know? What can you do with hemp that has been used for phytoremediation?
First, a little bit about plant biology. Different species of plants have differing tolerance and sensitivity to accumulating metals from the soil. Generally, this is done through the roots where the metals are found in some readily absorbable and water-soluble form (like as a salt). Plants, thus, fall into one of three categories: excluders, indicators & accumulators. The majority of agricultural plants are indicators and the metal content of these plants generally increases as soil concentration of metals increase. Accumulators concentrate higher amounts of metals in plant tissue than indicators, and “hyperaccumulators” are those plants that can commonly grow on metal-bearing soils without symptoms of toxicity. Plants can store metals in either below ground (roots) and above ground (shoots, leaves, seeds) parts. Leaves tend to hold more metals than seeds, so in general, vegetables will extract more metals into above ground parts than cereal grains.
The process of phytoremediation can remove organic and/or inorganic pollutants (metals, pesticides, persistent organic pollutants) from contaminated soil, sludge, sediments and water. Many small communities in the US have used aquatic plants and constructed wetlands to treat wastewater. To be truly useful, a plant should be native, grow quickly, have extensive roots, high biomass yield and be able to adapt to various habitats and possess the ability to accumulate pollutants in aboveground parts.
EPA has noted that maize, sorghum, tobacco and lucerne are known metal accumulat ors and plants from the famililes Caryophyllaceae, Brassicaceae, Cyperaceae, Poaceae, Fabaceae and Chenopodioceae. Fast-growing trees (especially willows) are very promising phytoremediants due to their large aboveground biomass and effective use of cadmium and zinc. Maize has been noted as perhaps an ineffective species for cadmium and lead due to large amount of metal accumulation in roots (below ground biomass); however, some herbaceous species are good for lead removal (such as Indian mustard, rye grass, sunflower or smallwing sedge).
In 2006, a research group from the Czech Republic compared how different biomass crops (white sweetclover, red clover, safflower, curled mallow and hemp) performed in both container and field experiments to remove arsenic, cadmium, lead and zinc from soil. Safflower was the most efficient at removing cadmium and zinc from soil in containers and in field experiments. None of the plants tested removed appreciable amounts of arsenic or lead. Overall, safflower and curled mallow showed the greatest effectiveness. Cannabis sativa was not found to be competitive with these plants in field experiments for the removal of cadmium, lead and zinc.
A Chinese research group in 2009 looked a cadmium removal rates in a number of potential biomass/energy crops, including hemp. Hemp, flax, castor and peanuts were found more tolerant to cadmium than soybean sunflower, safflower and rapeseed. In terms of total cadmium uptake from soil, peanut removed roughly 5x more cadmium than hemp. Hemp is perhaps a poor candidate for removing cadmium as it mostly collects this metal in its roots, while safflower and field mustard (Brassica rapa spp.) collected much higher quantities of cadmium in above ground parts (shoots). Plants that collect more metals in the roots as opposed to shoots are less effective for phytoextraction and more useful for phytostabilization. Hemp appears to be an outstanding candidate for phytostabilization of cadmium in soil.
This is but one small example of the type of analysis that would be needed on individual metals to determine to best phytoextraction crop for that metal. While it has been noted that hemp is a hyperaccumulator of metals in popular press accounts, this is clearly not the case when looks at individual compounds.
Now, we turn our attention to the uses of hemp roots and shoots that have been used for phytoremediation applications. Most heavy metals found as contaminants in soil are not desired as inclusions in any product that will be consumed by humans or animals. In others words, hemp as a “mop crop” to pull metals out of soil (i.e. phytoextraction) cannot be repurposed as human food or animal feed. This is not just an issue for hemp grown on contaminated soils, though. Why?
In 2012, a Romanian research group examined the metal content of hemp seed and oil, which can include essential elements for human biology (such as magnesium, phosphorus, iron and manganese) as well as undesired metals (zinc and cadmium). This work showed that significant amount of iron, manganese, zinc and cadmium can result in seeds grown in light to moderate areas of contamination and the authors concluded than hemp may be useful for phytoremediation of iron, zinc and cadmium.
Clearly, additional careful research is needed to ascertain the ability of the hemp plant to extract specific metals from soils into specific parts of the plant. If metal contents in seed are below levels of health concern, then it may be possible to use that crop for human food or animal feed. It may be the case that hemp does not extract certain metals into above ground parts at appreciable levels, which could potentially be the case for arsenic and lead.
Sources used:
Mihoc, M., Pop, G., Alexa, E., & Radulov, I. (2012). Nutritive quality of romanian hemp varieties (Cannabis sativa L.) with special focus on oil and metal contents of seeds. Chemistry Central Journal, 6(1), 122.
Rezania, S., Taib, S. M., Md Din, M. F., Dahalan, F. A., & Kamyab, H. (2016). Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. Journal of Hazardous Materials, 318, 587–599. http://doi.org/http://dx.doi.org/10.1016/j.jhazmat.2016.07.053
Shi, G., & Cai, Q. (2009). Cadmium tolerance and accumulation in eight potential energy crops. Biotechnology Advances, 27(5), 555–561.
Tlustoš, P., Száková, J., Hrubý, J., Hartman, I., Najmanová, J., Nedělník, J., … Batysta, M. (2006). Removal of As, Cd, Pb, and Zn from contaminated soil by high biomass producing plants. Plant, Soil and Environment, 52(9), 413–423.
What is the reality of hemp cultivation in West Virginia?
“Corn won’t grow at all on Rocky Top, dirt’s too rocky by far… that’s why all them folks on Rocky Top get their corn from a jar”
We certainly have our share of rocks and hills (and corn liquor!) in West Virginia as well, but can we really grow hemp here?
A student at Marshall University has performed a “hemp suitability” analysis [Cannoy (2015)] as part of his graduate studies in the Department of Geography through use of GIS tools and empirical data on climate, precipitation, slope and land use. I have suspected something akin to the conclusion of the song quote above but had not seen any actual data and arguments to back this up … only parts of WV are ideal for growing hemp. But where? Why?
““A general assumption was that hemp cultivation areas would be plentiful in West
Virginia because of the favorable climate and existing vegetation cover, which has proven not to be the case.””
There are real-world limitations to growing hemp that are directly related to WV’s physical features. Elevation and slope are the most important (as everywhere in the state pretty much gets sufficient sunlight and precipitation).
The plant requires about 3,300 growing-degree days, which is supported everywhere in the state except the central area – which can support two annual hemp crops.
Cannoy (2015)
Elevation can impact fiber quality, so hemp grown for seed production is perhaps a better bet at higher elevations. So sorry, Davis [see list of highest cities in the USA], you might have to settle for seed production as opposed fiber production. Same for you Rocky Top.
And then there’s slope. We are the Mountain State after all and slopes greater than 35° are not “feasible to farm due to problems harvesting the product.” In fact, it is suggested in the analysis that farmers not use slopes exceeding 5% for fiber cultivation. The greater the slope, the smaller your potential for cultivating fiber biomass.
Cannoy sifted all the state data on slope and elevation, etc. together and tried to pinpoint the areas in the state that are suitable for hemp seed or fiber production. Here’s what he found:
- Total acreage in WV for raw agricultural hemp production is roughly 3.6 million acres, or 23.5% of the total area of the state (which is 15.5 million acres)
- When one factors in actual land use, this drops suitable acreage significantly – down to just under 3% (or 448,126 acres). Much of this 448k are in historic coal mining areas, where acid mine drainage is present. (This represents both an opportunity and a limitation.)
Cannoy (2015)
The analysis supports the historic knowledge that Appalachian soils and climate are ideal for the Cannabis plant and shows that West Virginia does have good potential to support a hemp-based industries to operate. However, it will require working hand-in-hand with local planners (city and County) and agricultural boards to maximize the potential for hemp cultivation on the local level. This is now made possible by the excellent local analyses presented in Mr. Cannoy’s thesis.
These data provided in the report argue for a distributed model of sustainable agriculture in the state whereby the amount of acreage that can be devoted to hemp production are local decisions, to be empowered by resources and interests driven at the local level. For example, let’s look at the Eastern Panhandle where I live.
Cannoy (2015)
Spots that meet altitude and slope requirements are pinpointed along with incorporated and potentially contaminated areas. This type of granular data is critical to empower local decision-making coalitions with the information they need to maximize hemp production on the regional level. Clearly, as Mr. Cannoy points out, urban planners are absolutely critical to this effort and the use of GIS tools such as these are critical to helping to grow our nascent industry. In addition, many localities in the state establish “Comprehensive Plans” and these are ideal vehicles to codify local interest and intent in hemp cultivation. Planners and local governments should work together to modify these plans to expand use of sustainable agriculture, such as hemp production.
There are indeed limitations to the study and other ways to interpret the data. As an advocate for the use of the Cannabis sativa plant as both a preventive medicine (to reduce inflammation and to protect the brain from its effects) and a way to combat treatable conditions (such as chronic pain, PTSD, addiction, severe pediatric epilepsy disorders, etc.), the potential for medicinal hemp applications may in fact greatly outweigh the potential for its use as a source of seed of fiber.
The presence of cannabinoids useful for medical purposes may not affected by slope, altitude, etc. and this supports the ability of patients to cultivate their own medicine on some 3 million acres in West Virginia.
Not bad. Let’s grow our future, West Virginia.
Source: “Green Gold- a Cannabis Sativa L. Lucis Suitability Analysis for West Virginia,” by Delbert Christopher Cannoy in partial satisfaction of a MS in Geography from Marshall University. December 2015.
CALL TO ACTION: Strategic Cannabis Conference - Eastern Panhandle
The Strategic Cannabis Conference Team seeks participants, exhibitors and organizers for a May 2016 event in the Eastern Panhandle of West Virginia
On St. Patrick's Day 2016, a new type of conversation was started in West Virginia. The dialog featured experts from the medical community, academic law community, academic research community, local business community and the international cannabis industry to present the case for embracing the medical and industrial uses of Cannabis sativa in the Mountain State.
We now wish to bring this dialog to the Eastern Panhandle and we seek all interested parties, but specifically we seek speakers to round out our program as well as any other participants, exhibitors and organizers from the:
- cannabis & hemp industries seeking to do business in West Virginia;
- other successful local businesses & entrepreneurs and those wishing to invest in future successful WV cannabis and hemp businesses;
- farmers and farming organizations that experience cultivating Cannabis sativa;
- technicians, scientists, laboratorians, engineers;
- regulators, lawmakers and politicians either holding office currently or running for office;
- and most importantly patients wishing to have their voice heard
SPONSORSHIP and EXHIBITOR opportunities are available. Please contact stratcannacon AT gmail.com.
Details and a "Save the Date" to be announced soon.
Press Release
Agri Carb Electric Corporation Announces Intention to Operate in West Virginia
ACE CORP CEO Don Smith announces new company at
WVU Law School Conference
Morgantown, WV March 18, 2016: Chief Executive Officer Don Smith II announced the presence of his new company, Agri Carb Electric Corporation (ACE CORP), to the attendees of the ground-breaking Strategic Cannabis Conference 2016, held March 17 at the West Virginia University College of Law. ACE CORP seeks to establish infrastructure in West Virginia to process industrial hemp grown in the Appalachian region and is currently seeking funding partners and collaborators to achieve its vision.
According to Erik Janus, Vice President of Scientific & Regulatory Affairs, “ACE CORP is West Virginia’s newest cleantech startup that seeks to demonstrate and market advanced carbon applications from a sustainable agriculture base and we think industrial hemp is the key to establishing a regional bio-based economy and to providing jobs and revenue in the Mountain State.”
ACE CORP will also be a presence at future offerings of the Strategic Cannabis Conference 2016 around the state over the next several months. For more information on the WVU event, see http://law.wvu.edu/strategic-cannabis-2016. For media inquiries, please contact Mr. Janus at erik.janus@gmail.com or J. Morgan Leach, Lead Counsel, at moleach09@gmail.com.
This Week By the Numbers in West Virginia
This Week By the Numbers in West Virginia
This week …
1.15 veterans from West Virginia will commit suicide.
This week …
1.35 people under 25 in West Virginia will die of a drug overdose.
This week …
12 West Virginians will die of a drug overdose.
This week …
63 West Virginians will leave the state.
This week …
70 West Virginians will get arrested for possession of cannabis.
This week …
Another 48,582 opioid pills will get prescribed in West Virginia.
Upgrades, Newbies and Watch-Outs: Medical Cannabis Reform in the 2016 Legislative Session
There’s a fair amount of motion among the states with regard to medical cannabis this legislative session. Here’s a spectrum of the most notable from the Upgrades (going from “CBD-only” to medical cannabis legislation) to the Notorious Nine. Only those states with bills in both chambers are considered – Nebraska being the only state that does not have a bicameral legislature.
In West Virginia, there are multiple bills under consideration:
· Senate Bill 640: The Compassionate Use Act for Medical Cannabis, by Senators Kessler, Carmichael, Stollings, Prezioso and Plymale.
· House Bill 4680: Creating a medical exemption to criminal laws against marijuana use and possession for patients as well as protections for doctors, by Delegates Flanigan, McGeehan, Folk, Wagner, Sponaugle, Eldridge, Skinner, Hornbuckle, Storch, Ihle and Pushkin.
· House Bill 4712: Provides for decriminalization, sets up a taxation system and penalties for enforcement and removes cannabis from the Uniform Controlled Substances Act, by Delegate Pushkin.
Together, they form the basis of a NEW regulatory program that would provide medical cannabis to registered and qualified patients through “compassion centers,” with your doctor’s approval, for conditions such as cancer, glaucoma, PTSD, opiate addiction and chronic pain. There’s even a provision in the bill to add other treatable conditions as medical research uncovers yet more ways to use medical cannabis to treat disease.
Other than the Mountain State’s efforts – which of course get prominence as these are the bills I have been supporting this session – here is the rest of the pack.
Upgrade States!
· Florida
http://medicalmarijuana.procon.org/sourcefiles/florida-ballot-measure-2016.pdf
http://medicalmarijuana.procon.org/sourcefiles/florida-SB852.pdf
http://medicalmarijuana.procon.org/sourcefiles/florida-hb1183.pdf
· Missouri
http://medicalmarijuana.procon.org/sourcefiles/missouri-sb912-2016.pdf
http://medicalmarijuana.procon.org/sourcefiles/missouri-hb2213-2016.pdf
http://medicalmarijuana.procon.org/sourcefiles/missouri-sjr29-2016.pdf
· South Carolina
http://medicalmarijuana.procon.org/sourcefiles/south-carolina-sb-0672-2015.pdf
http://medicalmarijuana.procon.org/sourcefiles/south-carolina-hb4003-2016.pdf
http://medicalmarijuana.procon.org/sourcefiles/south-carolina-hb-4307-2015.pdf
http://medicalmarijuana.procon.org/sourcefiles/south-carolina-hb-3140-2015.pdf
· Tennessee
http://medicalmarijuana.procon.org/sourcefiles/TN-SB-660-2015.pdf
http://medicalmarijuana.procon.org/sourcefiles/tennessee-hb-561-2015.pdf
Of the remaining Notorious Nine, only five states have had bills introduced this legislative year.
Newbie States!
Pennsylvania: Possession of less than 30 grams is a misdemeanor with 30 days in jail, while over 30 grams gets you a year. Cultivating even one cannabis plant is a felony with 1 to 5 years in prison.
http://medicalmarijuana.procon.org/sourcefiles/pennsylvania-sb3-2015.pdf
http://medicalmarijuana.procon.org/sourcefiles/pennsylvania-hb193-2015.pdf
Kansas: Surprisingly, up to almost a pound of marijuana is just a misdemeanor with a year incarceration, but only for the first offense. For a second offense, any amount of marijuana is a felony with 10 months to 3.5 years in prison.
http://medicalmarijuana.procon.org/sourcefiles/kansas-hb2011.pdf
http://medicalmarijuana.procon.org/sourcefiles/kansas-sb9-2016.pdf
Indiana: Possession of any amount of marijuana is a misdemeanor worth six months in jail, and a single cannabis plant is worth a year.
http://medicalmarijuana.procon.org/sourcefiles/indiana-sb209.pdf
http://medicalmarijuana.procon.org/sourcefiles/indiana-hb1284.pdf
Nebraska: Nebraska has more lenient laws relative to these others with a limited degree of decriminalization.
http://medicalmarijuana.procon.org/sourcefiles/nebraska-lb-643-2015.pdf
These states are not even trying … and HERE is where it will get you.
Watch-Out States!
North Dakota: Not only is possession of any amount of marijuana worth 30 days in jail, but merely ingesting hash or concentrates is worth a year in jail. Possessing any hash/concentrate or more than an ounce of marijuana is a felony with 5 years in prison.
Louisiana: Possessing up to a half-ounce gets you 15 days on your first offense. Get caught a second time, and you’re looking at six months in jail. Any cultivation of cannabis earns you a mandatory minimum 5 years in prison for a first offense.
Arkansas: Possession of any amount of marijuana can land you in prison or parole for up to a year.
Idaho: Not only is possession of less than 3 ounces a misdemeanor worth 1 year in jail and a cannabis plant a felony with a 1 year mandatory minimum in prison, but Idaho will lock you up for 3 months for merely being somewhere that somebody stores marijuana. Idaho will also lock you up for 6 months for merely being under the influence of marijuana in public.
South Dakota: Probably the most dangerous place for a medical marijuana patient in America. While possession of less than 2 ounces is a misdemeanor with a 1 year sentence, authorities need only find marijuana metabolites in your urine to get that conviction, as South Dakota has the nation’s only “internal possession” statute. Like Idaho, you can get time for being in a place where marijuana is stored, but it’s a full year in a South Dakota jail rather than Idaho’s three months. Plus, possession of any amount of concentrate is a felony that will get you a decade behind bars.
In Pennsylvania, it almost seems certain their legislation will pass, after ignoring it for weeks. Might it be related to the Governor's cancer? The suite of bills in front of the West Virginia legislature doesn't look hopeful, and the Senate in Indiana will vote in March. The Kansas senate passed a decriminalization bill, but the medical cannabis bill status is still winding through. In Nebraska, the Attorney General stands in opposition to medical cannabis.
Things could change again in the fall with voter referendums ... stay tuned.
Should the industrial hemp industry fold to federal pressure regarding cannabidiol?
A concerted effort by the federal government is putting significant pressure on the cannbidiol market. Will it work?
Cannabidiol (CBD) is the non-psychoactive cannabinoid cousin of delta-9-tetrahydrocannibinol (THC, the molecule mainly responsible for the cannabis-associated "high") and the market for it has simply exploded since the reinvigoration of the global industrial hemp market.
The molecular structure of cannabidiol (CBD).
What do we know about CBD?
CBD is not THC, as this infographic will show you. In short, the retail cannabis marketplace thrives on short squat female plants that produce 20-30% THC in the resultant flowers while the industrial hemp marketplace thrives on (mostly) tall lean males that contain minute quantities of THC and can be harvested for long, strong fibers (for example). Most regulatory agencies around the world consider anything with less than 1% THC industrial hemp (non-drug-type Cannabis sativa); however, most technically define hemp as 0.3% THC or less and, practically, the levels of THC found in hemp strains grown today are much less than this. The amounts of CBD can vary in both drug-type Cannabis sativa (grown for medical and retail markets) and non-drug-type industrial hemp. Often, retail cannabis has negligible levels of CBD, followed by medical-grade cannabis and hemp - both of which have strains that where CBD can reach into the 20-25% range.
Charlotte's Web is a well-known low-THC, high-CBD strain of Cannabis sativa.
CBD does not possess psychoactive properties; however, it is *the* driving factor right now for the medical uses of cannabis. Its use to treat special forms of childhood epilepsy that are resistant to conventional treatment has been nothing short of spectacular. This is evidenced by the fact that forty (40) states now allow patients access to CBD, as opposed to just 23 and the District of Columbia who allow patients access to THC via drug-type Cannabis sativa.
CBD has been demonstrated to provide a spectrum a health benefits due to its ability to act as a strong antagonist (i.e. blocker) of the endocannabinoidome (ECD) receptors. The ECD is the extended view of how the research community now views the endogenous cannabinoid signaling system found in man (the "endocannabinoid system," similar to the enkephalin system and its series of opioid receptors through which the effects of endorphins, morphine, heroin and synthetic opioids (like Percocet, Oxycontin, etc.) etc are felt.
An exhaustive view of CBD pharmacology and its effects are beyond the scope of this article, but the reader is referred to Welty, T. E., Luebke, A., & Gidal, B. E. (2014). Cannabidiol : Promise and Pitfalls. Epilepsy Currents, 14(5), 250–252. Some discoveries that have been made recently include:
Over 65 discrete targets have been reported in the literature for CBD, however, treatment of neurological disease associated the regulation of intracellular calcium levels shows promise. Ibeas Bih, C., Chen, T., Nunn, A. V. W., Bazelot, M., Dallas, M., & Whalley, B. J. (2015). Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics, (AUGUST).
Zhornitsky and Potvein (2012) reviewed 18 randomized clinical trials in human populations, including multiple sclerosis (six studies), schizophrenia and bipolar mania (four studies), social anxiety disorder (two studies), neuropathic and cancer pain (two studies), cancer anorexia (one study), Huntington’s disease (one study), insomnia (one study), and epilepsy (one study). Zhornitsky, S., & Potvin, S. (2012). Cannabidiol in humans-The quest for therapeutic targets. Pharmaceuticals, 5(5), 529–552.
- CBD may have utility in treating human diseases and disorders known to involve activation of the immune system and associated oxidative stress, such as rheumatoid arthritis, types I and II diabetes, atherosclerosis, Alzheimer’s disease, hypertension, the metabolic syndrome, ischemiareperfusion injury, depression, and neuropathic pain. Booz, G. W. (2011). Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radical Biology Medicine, 51(5), 1054–1061.
- Preclinical (animal) data appear to support a positive role for CBD treatment in the heart, and in peripheral and cerebral vasculature. Stanley, C. P., Hind, W. H., & O’Sullivan, S. E. (2013). Is the cardiovascular system a therapeutic target for cannabidiol? British Journal of Clinical Pharmacology, 75(2), 313–322.
- CBD might reduce seizure frequency and might have an adequate
safety profile in children and young adults with highly treatment-resistant epilepsy. Devinsky, O., Marsh, E., Friedman, D., Thiele, E., Laux, L., Sullivan, J., … Cilio, M. R. (2015). Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. The Lancet Neurology, (January 2016).
GW Pharmaceuticals is perhaps the biggest player in the CBD drug development market and not only commercialized the world’s first cannabis extract prescription drug (a mix of THC and CBD, Sativex, for the treatment of spasticity due to MS), but has also "Orphan Drug Designation" from the FDA for Epidolex, a pure CBD drug, for the treatment of Dravet and Lennox-Gastaut syndromes, severe, drug-resistant epilepsy syndromes. GW’s product pipeline also includes compounds in Phase 1 and 2 clinical development for glioma, ulcerative colitis, type‑2 diabetes, and schizophrenia.
CBD products and the CBD "gold rush" is big business and the federal and state governments knows it. The fledgling industrial hemp market is looking to produce CBD on a large-scale from non-drug-type hemp plants that are legal in those states that have regulated markets (about 2 dozen, including West Virginia, Kentucky and Colorado).
Hasn't this come up before?
Absolutely - like here for example. In HIA v DEA (2003), the DEA tried to write rules and related retroactive interpretations of the Controlled Substance Act impacting certain cannabinoids that were expressly covered under the original intent of the law. While the court case told the DEA it could not go back and change the intent of the law, it still maintains the position that CBD is a Schedule 1 substance with no medical benefit, just like marijuana.
The confusion will remain as CBD is not specifically covered or defined under the CSA.
What is the government doing?
It appears that both the state and federal governments have initiated a concerted effort to quell the raidly expanding CBD market. Reasons are not clear but evidence is abundant. In addition to the continued pressure that DEA's questionable policy on CBD's legality brings to bear, both the Food & Drug Administration (FDA) and Customs & Border Patrol (CBP) have stepped up enforcement efforts.
FDA: Despite on-going discussion between law firm Hoban & Feola LLC and the FDA regarding nationwide retail placement of CBD products, FDA posted new information on its website regarding medical cannabis in May 2015. In this non-binding, informal "Q&A" document posted online, FDA declared CBD products are excluded from the definition of "dietary supplement" as found in the Federal Food, Drug and Cosmetic Act (FFDCA). As a follow up to this industry notice, FDA issued warning letters to several companies marketing dietary supplement CBD products in February 2016. First and foremost, FDA took issue with the unsupported health claims made on the packaging of these products. Next, FDA claimed "CBD Oil" is not allowable as an ingredient due to previous investigation as a drug. In other words, another company submitted an official application to FDA for use of CBD Oil as a drug.
CBP: Starting in August 2015, there was an uptick in imprted hemp good seizures at the border. According to Hoban & Feola, these were not announced, records were not kept and collective finger-pointing was left these early seizures "unresolved." CBP claimed these were illegal dietary supplement imports and referred to FDA for action, but none was taken and the shipments were released. Later that year, CBP started testing for THC concentration and using its federal authority instead. By November, CPB issued a "zero tolerance" policy statement, citing the DEA policy that HIA had the court invalidate in 2003. During 2015, CBP went from a position of allowing up to 0.3 THC into the country to allowing NO, NONE, ZERO, ZILCH in a CBD product.
Why the hemp industry should press ahead...
While the current situation and pressure coming from the federal government is serious, it is also stands on shaky ground. The DEA's position on CBD is tenous and could be rendered moot with a simple legislative fix, the FDA hasn't issued any binding guidance on this and its interpretation could be (and has been) challenged, and the CPB logic is, quite simply, bizarre if not poorly justified.
The simple legislative fix I refer to stands before Congress now and has enjoyed mulitple trips to Congress since 2009 - currently known as the Industrial Hemp Farming Act of 2015. It would simply amend the CSA to exclude industrial hemp from the definition of "marijuana." It would define hemp as no more than 0.3 % THC. It would free the industrial hemp market from the shackles it has been in since the 1930s and allow many folks to participate in a new and growing agricultural market.
FDA's guidance to date consists of an online "Q&A" and some warning letters. These would not be considered actions binding on the regulated community. The letters invite the regulated community to submit data proving the validity of existing market claims and some companies, like CV Sciences (formerly CannaVest), have done so and pointed out the confusion that remains with the FDA decision. It should also be pointed out that other dietary supplements still on the market today have received a equal magnitude of scrutiny from FDA. Finally, Hoban & Feola point out that in no way has FDA made a determination that CBD products are illegal or non-compliant with CSA -- as both DEA and CPB have.
I believe this attack on the industrial hemp market will pass; however, for now, one is very wise to consult heavily with lawyers and consultants moving into 2016.
Developing cannabidiol-based treatments for chronic pain and mental health disorders
Innovative Holdings Alliance, Inc. and Synaptic OTC Sciences Innovative have announced a joint venture to develop "nutraceutical products and therapies aimed at providing relief for a number of diverse ailments and maladies, including but not limited to chronic pain, sleep disorders, depression, Post-Traumatic Stress Disorder (PTSD) and Social Anxiety Disorders (SAD)."
Through this JV, Innovative will provide managerial, financial and marketing expertise to license Syanptic's portfolio of cannabidiol (CBD) and hemp products, with an initial focus on treatments for chronic pain. Cannabis sativa has been used for thousands of years to control pain and several recent systematic reviews have found its use safe and effective.
In addition to the enormous cost to the health care industry and patients, there is has been an enormous societal toll to increased access to opioids, particularly in certain states like New Mexico and West Virginia. Medical cannabis can treat all types of pain and offers a much lower safety profile than opioids -- the only other course of treatment for chronic pain -- and with little risk of developing tolerance. In fact, a 2015 case report described the remarkable siutation of a man who, after getting a new liver, weaned himself down from 30-40 milligrams of opioids per day to 6-8 milligrams per day with the help of medical cannabis to control his severe post-operative pain.
These CBD-based treatments will be a boon to sufferers of chronic pain, which in many cases, include our nation's veterans, who only have partial access to medical cannabis due to federal restrictions on its use.
Looking at some of the evidence supporting veterans' use of medical cannabis
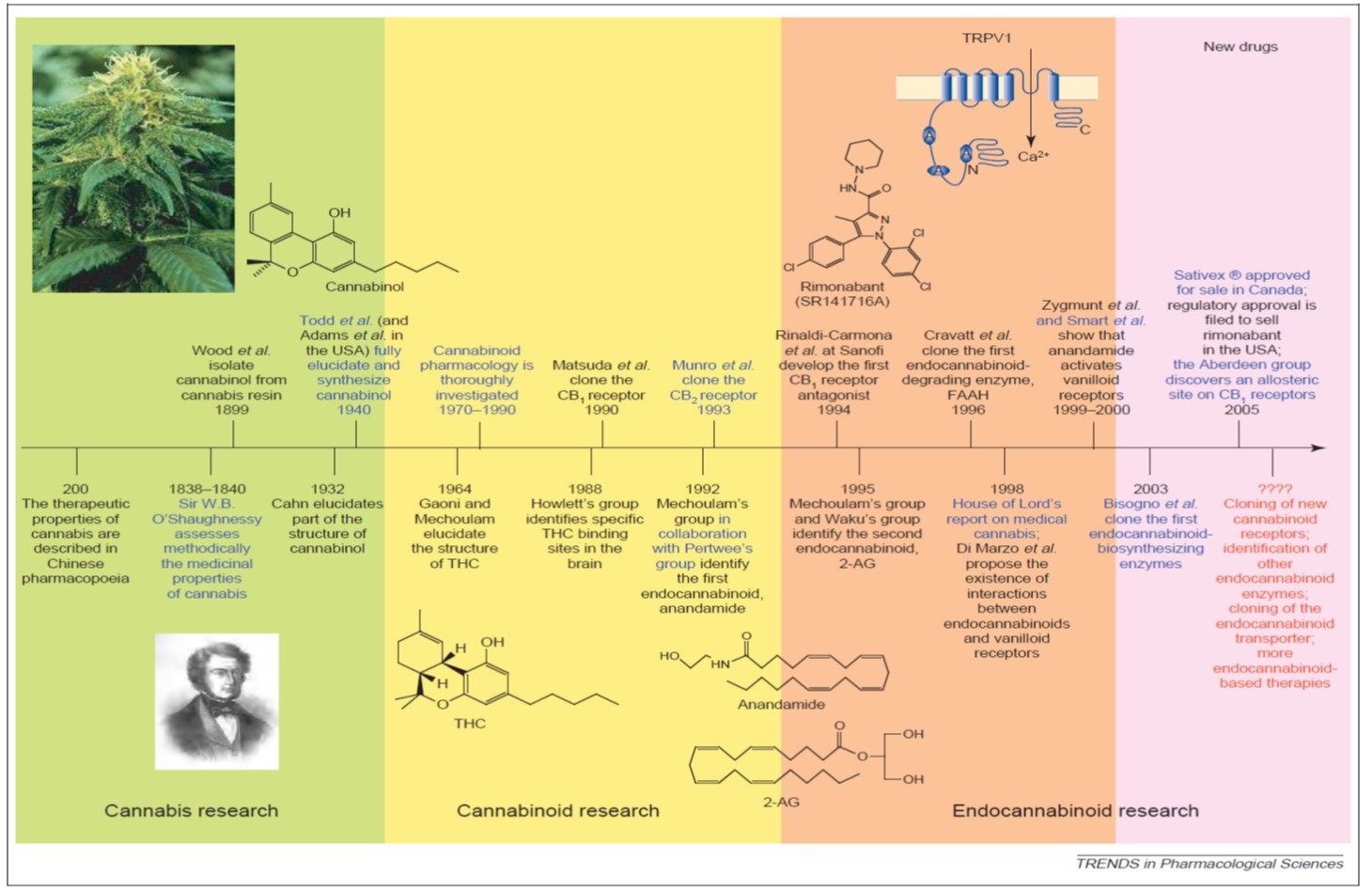
Cannabis sativa is a plant that man has cultivated for both its medicinal as well as its psychoactive properties for many thousands of years. (You may have heard of hemp as well, but that's a much longer story ...) It is indigenous to temperate regions of Asia, so its earliest known use as a medicine traces back to ancient Chinese and Indian civilizations. In the 1800s, "Cannabis indica" (aka Indian hemp, Indian cannabis) was introduced into Western medicine primarily by an Irish physician named William O'Shaughnessy (who visited Calcutta in the 1840s) and was a common ingredient in every physician's kit bag by the early 20th century.
By 1851, it had been added to the US Pharmacopeia (removed by the 1940s) and there were many medical studies and scientific papers published during this time, culminating in the first conference on its use as a medicine held in 1860 by the Ohio State Medical Society. Cannabis fell out of use in medicine by the 1930s for many reasons, not the least of which was the introduction of morphine and the hypodermic needle - both of which became immediately more useful to control pain, for example. Oh, and a little thing called the Marihuana Tax Act of 1937 (read about the history of this).
One can peruse a compressed timeline of key research events above; however, there have been at least two publication surges since the 1800s. The first surge was in the 1960s when THC and cannabidiol (CBD) were discovered and characterized and the second was in the 1990s, when the early discoveries of the endogenous cannabinoid signaling system ("endocannabinoid system," or ECS) were published. Since then, there has been a a slow, steady and sustained increased in annual publications in the field of cannabinoid research from roughly 300 articles per year to over 2,000 per year today (my research, data not shown or published).
One simply cannot underestimate the importance of the ECS to human biology. While there is still much to learn about the system, our current expanded view of this retrograde signaling system is referred to as the "endocannabinoidome," or ECD. It has been found to operate in virtually every tissue and organ system in the body that has been investigated. As one can see below, the ECD is composed of a series of receptors (CB1, CB2, TRPV1 and several GPRs), endogenous signalling molecules (or "endocannabinoids" [eCBs] like anandamide ... there are 8 others as well), membrane transporters and enzymes to break down the eCBs once their job is done.
More about the ECD:
- It has been shown to play key role in “fine tuning” metabolic and homeostatic mechanisms (It makes sure everything runs smoothly from a biochemical point of view).
- It has links to and potential signalling roles in the immune system, digestive system, cardiovascular system, nervous system, skin and skeletal system (for starters!!).
- It plays a key role in chronic disease, including tissue degeneration and remodeling (in bone), inflammation (including in the brain, which serves a neuroprotective role) and in controlling all types of pain.
- It is interconnected with other lipid and non-lipid signaling systems (and other regulatory networks) in a very complex and still not-quite-understood manner.
The cannabinoids (like THC, CBD, etc.) found in Cannabis sativa are referred to as phytocannabinoids (pCBs) and synthetic derivatives (synthetic cannabinoids, sCBs) have been prepared in the laboratory and approved by FDA as drugs. There are essentially three sCBs on the market: Marinol, Cesamet and Sativex. The first two are 100% synthetic THC (in pill form) and the final is a pCB extract, formulated as a sublingual (under the tongue) spray. Another drug currently under investigation is Epidiolex, which is 100% CBD and being examined in clinical trials with children with intractable/severe epilepsy. In fact, many states have now allowed the use of CBD oil to treat pediatric epilepsy that is resistant to conventional treatments. Extracts hold great promise for research due to the "entourage effect," which is the phenomenon described in the literature where more benefits are found from administration of cannabinoids and terpenes together (also found in Cannabis sativa ... about 150 are known to exist and these impart the smell and taste to the plant's flowers and resin) than from THC alone.
Many states have now also instituted state-based programs that regulate the cultivation, processing, distribution and dispensing of medical cannabis products to patients. Patients going through the Veterans Administration health care system; however, generally do not have access to cannabinoid medications due to federal law and policy conflicting with these state-based programs. This is unfortunate as there are many common conditions that our nation's veterans present to VA physicians with, some of which are resistant to conventional treatments. Most states with a medical cannabis regulatory program maintains a list of "treatable conditions" for which cannabinoids may prescribed. (Maryland comes to mind as a notable exception, a state that will not publish such a list and will rely upon judgments made by state-registered physicians engaged in a "bona fide" doctor-patient relationship.)
Some of these conditions include:
- Pain control
- Both human and animal studies show that cannabinoids act as "analgesic" agents and the efficacy of individual products (how well it works) is variable & depends on route of exposure (i.e. oral, inhalation). Analgesic effects have been noted for neuropathic pain, inflammatory pain and cancer pain. Cannabinoids act synergistically with opioids -- currently, the only analgesic for treating severe and chronic pain -- and can act as an “opioid sparing agent” (lower doses, fewer side effects) while having an improved safety profile (i.e. NO OVERDOSES from cannabinoids. Much lower potential for abuse.)
- A systematic review of clinical trial data for treating chronic non-cancer pain from 2011 stated: "Overall the quality of the trials was excellent. 15 of 18 trials that met the inclusion criteria demonstrated a signfiicant analgesic effect of cannabinoid as compared to placebo and several reported significant improvements in sleep. There were no serious adverse effects." [Lynch, M. E., & Campbell, F. (2011). Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. British Journal of Clinical Pharmacology, 72(5), 735–744. http://doi.org/10.1111/j.1365-2125.2011.03970.x]
- A systematic review from 2015 for treatng chronic non-malignant neuropathic pain stated: "Cannabis-based medicinal extracts used in different populations of chronic nonmalignant neuropathic pain patients may provide effective analgesia in conditions that are refractory to other treatments." [Boychuk, D. G., Goddard, G., Mauro, G., & Orellana, M. F. (2015). The Effectiveness of Cannabinoids in the Management of Chronic Nonmalignant Neuropathic Pain: A Systematic Review. Journal of Oral & Facial Pain and Headache, 29(1), 7–14. http://doi.org/10.11607/ofph.1274]
- The largest and most recent systematic review (looking at all randomized clinical trial data available for cannabinoids) from 2015 stated: "Compared with placebo, cannabinoids were associated with a greater average number of patients showing a reduction in pain" from 8 trials, which the authors characterized as "moderate-quality evidence." [ Whiting, P. F., Wolff, R. F., Deshpande, S., Di Nisio, M., Duffy, S., Hernandez, A. V., … Kleijnen, J. (2015). Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA, 313(24), 2456. http://doi.org/10.1001/jama.2015.6358]
- Inflammation control
- All classes of cannabinoids show anti-inflammatory activity and the role of THC and the endocannabinoids in this response is well-studied. CBD has been shown to reduce joint, instestinal and neuroinflammation in animal models (including reduction of glucose-induced inflammation in diabetic mice). At least one terpene (caryophyllene) has been found to have anti-inflammatory activity and can bind to one of the ECD receptors. There's even some evidence in acute brain injury models that cannabinoids serve a neuroprotective role by reducing swelling.
- Post-Traumatic Stress Disorder (PTSD)
- PTSD patients have been found to express more CB1 receptors in their brain and thus, the stimulation that cannabis provides at this receptor has been widely found to provide effective short-term relief of symptoms. What is less well known are the long-term impacts as well as the impacts of self-medication and not adhering to prescribed doses. One small clinical trial of 10 patients in 2014 found that cannabis was well-tolerated and reduced symptoms of hyperarousal; however additional clinical trials are ongoing and/or planned. The VA system has reported in a series of publications over many years that, among veterans (seen in VA health care facilities) with co-occurring PTSD and substance use disorders (SUD), cannabis use disorder (CUD) is most diagnosed SUD since 2009. So there appears to be a fine line between controlling symptoms in the short-term and CUD in the long-term. In the meanwhile, CBD is also now being explored as a way to control social anxiety.
- A 2015 study from the VA reported that: "There is convincing evidence from multiple studies for reduced endocannabinoid availability in PTSD." (indicating endocannabinoid-targeted therapies should be explored) [See "Translational evidence for a role of endocannabinoids in the etiology and treatment of posttraumatic stress disorder."]
- Addiction
- This is one of the newer areas of research and both THC and CBD have been investigated for use in treating addiction. Studies done in the last decade have found cannabis appears to reduce cravings for several highly addictive substances, like crack cocaine, opiates and alcohol. Users claim less withdrawal symptoms, fewer side effects (than from conventional addiction treatment substances, like buprenorphine or methadone) and better symptom management. A systematic review of available evidence in 2015 found that some human studies have demonstrated benefit from CBD in treating cannabis and tobacco dependence. Sativex (a blend of equal amounts of THC and CBD) was even found to attenuate cannabis withdrawal symptoms in a 2014 clinical trial and ongoing trials in the US and Canada are investigating use of CBD in treating cocaine, opiod and cannabis addiciton. One very interesting case report from 2015 found a Canadian medical cannabis patient was able to fairly quickly taper down from 30-40 mg of opioids a day (prescribed for post-operative pain following a liver transplant) to 6-8 mg/day by using medical cannabis to control his chronic and severe pain as well as nausea. For pain, a strain with 17% CBD and less than 1% THC was used and for nausea, a strain with 12% CBD and 9% THC was used. [Meng, H., Hanlon, J. G., Katznelson, R., Ghanekar, A., McGilvray, I., & Clarke, H. (2015). The prescription of medical cannabis by a transitional pain service to wean a patient with complex pain from opioid use following liver transplantation: a case report. Canadian Journal of Anesthesia/Journal Canadien D’anesthésie, (January 2016). http://doi.org/10.1007/s12630-015-0525-6]
- Cancer
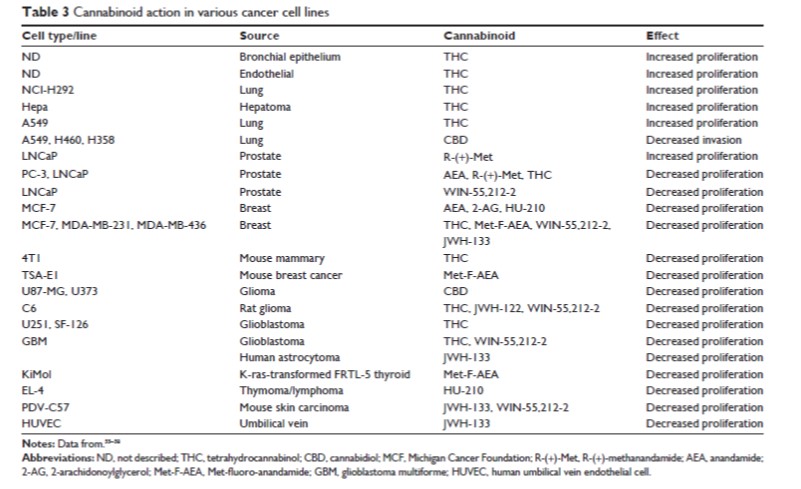
- The first study to be published that showed anti-tumorigenic properties of cannabinoids was in 1975! Since then, palliative care applications have long been recognzied and cannabinoids have played an important role in controlling pain, nausea, vomiting and appetite in cancer patients. Both in vitro and in vivo evidence support the hypothesis that endogenous cannabinoids can reduce proliferation and invasivity of prostate cancer cells; however, there has been much research in a wide variety of cell types and there have been mixed results. Some results can be seen below ... it is worth pointing out that THC itself has been to have both pro-profliferative and anti-proliferative properties.
While the results presented above are promising, they represent but a sliver of the available evidence. It must be stated strongly that additional mechanistic work is needed in animal, in vitro and human systems to more fully understand the role the ECS plays in maintaining "wellness." Even more important is the need for federal funding to run large-scale clinical trials in groups of human patients to understand more fully all the safety and efficacy issues for cannabinoid-based therapies. At this point, large cannabis industry companies like Tilray are funding larger-scale trials. The key to restoring FDA's ability to conduct large-scale trials is the removal of cannabis from the Controlled Substance Act scheduled list of illegal substances with no medical value ... a move that should happened a long time ago.
Essential Reading (all are free downloads):
Modulating the endocannabinoid system in human health and disease: successes and failures (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3684164/)
Re-branding cannabis: the next generation of chronic pain medicine? (http://www.futuremedicine.com/doi/pdf/10.2217/pmt.14.49)
Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4444130/)
Cannabis - the Israeli perspective. (http://www.ncbi.nlm.nih.gov/pubmed/26426888)
Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. (http://www.ncbi.nlm.nih.gov/pubmed/21749363)
Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4301686/)
Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3481523/)
Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4707667/)
Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3951193/)
Infographic: "What's in that weed?"
Please take and use this infographic for educational purposes. Please leave the attribution and logo. Enjoy!
Cultivation of hemp and cannabis offers American agriculture a "do-over"
Anyone who has paid attention to the snowballing liberalization of state law addressing the cultivation use of Cannabis sativa (cannabis) as industrial hemp, medicinal cannabis or the recreational may already know this ... but ... this a real game-changer, folks! But maybe not in the way you might you have thought.
First, this is a fantastic new market(s) opportunity for many small business owners that otherwise might have not have such market access. Perhaps more importantly, that greater adoption of cannabis and hemp cultivation offers society the moment to re-think policy and law in a number of areas affecting sustainable agriculture: energy and water use, the use of crop protection products and Integrated Pest Management strategies and the overall regulatory process. (There are, of course, so many more considerations, such state banking, tribal sovereignty, bankruptcy, IRS reporting etc.) These choices are important as they can result in the balance between black market activity and well-regulated safe market activity.
Much of the work affecting these areas of sustainable cannabis & hemp cultivation is ongoing (or soon will be) in California, where Governor Jerry Brown signed a trio of landmark bills that overhaul and replace the now 20-year old Proposition 215 (Compassionate Use Act) - the first medical marijuana regulation of its kind in the United States when first enacted in 1996.
Energy and water use
Cannabis sativa originated several thousands of years ago in water-rich temperate areas of Central Asia and the Indian subcontinent. It's a fairly water-hungry plant of variable dimensions (3 to 15 feet), dependent on growing conditions and agricultural practices, so getting accurate numbers regarding overall water use is difficult. (I've seen estimates spanning 1 to 10 gallons per plant per grow, which is typically about 90 days.) Scott Bauer and a team of people from the California Department of Fish & Wildlife (CDFW) released a study that found already-low streams in the three Humboldy County and one Mendocino County watersheds they studied were reduced volumetrically another 23% from illegal grow operations in the area from diverting stream water. Although the estimates of water usage were from remote image sensing (and not actual water meters), this water diversion was found to have an effect on the amphibious environment as well as particular (endangered ... whoops) species. Cannabis and hemp crops may use up to 2 - 2.5 times the amount of water that a wine grape crop would, and there are 10 times more pot farms (~ 50,000) than wineries (~ 4,000) in the state of California.
California initiated a pilot project run by the State Water Resources Control Board (Water Boards) and CDFW to address these issues in the North Coast region. The Water Boards will develop a regulatory program to protect water from "harmful activities from cannabis cultivation" - the new state bills AB 243 and SB 643 direct the Water Boards and CDFW to protect instream fish spawning, migration, and rearing from the damaging impacts of outdoor cannabis cultivation - by prohibiting waste discharges from agricultural practices, land clearing and grading activities in rural areas and forests and issuing permits (and collecting fees most likely) that cover medical cannabis grown on private land.
These are but a few examples of areas in which state departments of agriculture can work with the legislature and all other stakeholders to develop modern sustainable approaches to the cultivation of cannabis & hemp.
Pesticide use
As the opening graphic above portends, the choices we make regarding law, policy and regulation affect the balance between black market activity and safe, well-regulated market activity. Let's consider the use of "crop protection products" - which include everyone's favorite most-hated word pesticides (i.e. chiefly, herbicides, insecticides and fungicides) but also plant growth regulators, inoculants, antimicrobials and biopesticides). Currently, the balance is way off and there is little *but* black market activity by all indications. Of course, technically use of any EPA-regulated pesticide against its label use is illegal (and thus black market) and no pesticide labels currently include use on cannabis or hemp. (Although one can use substances that are not regulated by FIFRA through any number of exemptions, such as the use of minimum risk pesticides.)
EPA has offered a road map on how to achieve an actual label - through the use of the FIFRA Special Local Needs process - it is not easy, requires partnership with a current EPA pesticide registrant (think Big Ag companies like Dow, Syngenta or BASF) and would likely require some degree of data generation. There may be some motion towards this at the Colorado, Oregon or Washington ag departments (which are the co-enforcers of FIFRA along with EPA), however, anyone going down this road hasn't made it public yet. ( ... and anyone and everyone interested in trying to register pesticides for use on cannabis and hemp should get in touch with me!)
The obstacle towards greater EPA consideration and instruction (and resources!) is, of course, the current DEA Schedule 1 classification. That's also why the BigAg/pesticide companies don't want to even think about it right now ...
Reproduced from Small and Marcus (2002)
Regulation of agriculture for the small entrepreneur
There's perhaps never been a greater potential for inclusion in the marketplace for small business owners than the approaching hemp market. This strikes me as the ideal opportunity to address regulatory issues that may impede this community. Not only is small business the bedrock of the US economy, it helps build community.
Cultivation of hemp for any of 25,000 uses (by some estimates) is no small order and there will be more small farmers doing it than ever before. And they will have small budgets. And they will need protection and education in a broad array of areas. There will simply not ever be enough resources to police this veritable army of hemp farmers that could result from continued liberalization of state laws, particularly if the legislative trend to limit production to one acre for certain small-scale producers holds across much of the country. (That's a lot of one-acre hemp plots....)
Protection and education comes at a price however and states should be prepared to budget the dollars to implement successful cultivation conditions. This includes enforcement. Even though counties such as Medocino have passed their own laws regarding licensure, the enforcement of such laws can often be left to others - in this case, the CDFW and Water Boards.
Again, these are just some of the regulatory areas that state departments of commerce and law can work with the legislature and all other stakeholders to foster conditions for modern sustainable approaches to the cultivation of cannabis & hemp.
“… each family shall have a plot of not more than (40) acres of tillable ground, and when it borders on some water channel, with not more than 800 feet water front, in the possession of which land the military authorities will afford them protection, until such time as they can protect themselves, or until Congress shall regulate their title.”
This 40 acres (the mule came later) is what Sherman and Secretary of War Edwin M. Stanton pffered former slaves. What can we offer ourselves in terms of a "do-over" for American agriculture?
How many states have considered allowing the use of medical cannabis and cannabidiol oil?
Information accurate as of October 26, 2015
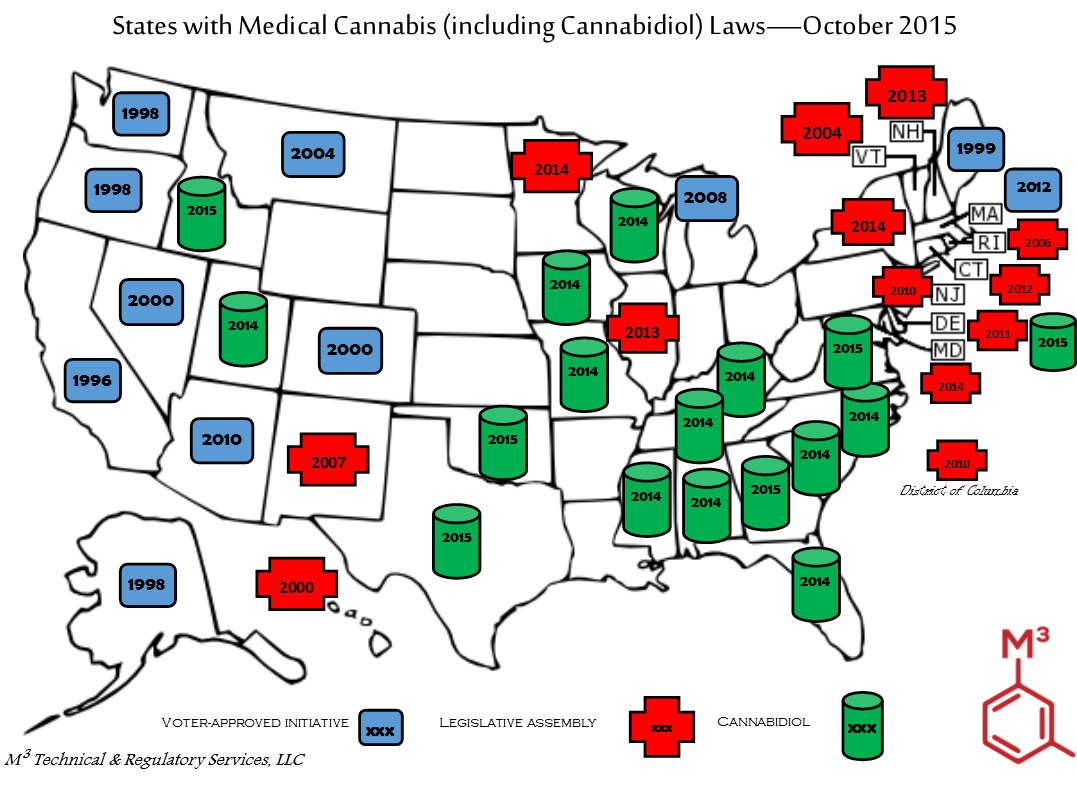
Since 1996, eleven (11) states have approved the used of medical cannabis via voter-approved initiatives (i.e. 1996, Proposition 215 in California).
Since 2000, twelve (12) states and the District of Columbia have approved the used of medical cannabis via legislative assembly.
Since 2014, sixteen (16) states allow access to cannabidiol oil, generally for a short list of severe conditions that are medically difficult to cure or provide relief for.
A trio of bills was passed via legislative assembly was passed earlier this month in California to overhaul the program established in 1996 via voter referendum.
Please feel free to download and use this high-quality graphic. Please leave my company logo and name.
How recent advances in toxicology will revolutionize the medical cannabis industry: Part 4
There are two major revolutions unfolding whose paths are about to cross … not only for the betterment of the cannabis and hemp industries but for the future of toxicology as well.
Part 4 : Two Roads Converged in the Woods ... ?
OK, perhaps it's not the woods. And this is not the classic poem by Robert Frost about roads diverging. But that's because we are not "out of the woods" yet when it comes to unlocking the molecular mode-of-action for the collection of conditions and diseases that phytocannabinoids may be able to treat. And the path converges far ahead down the road ... but it doesn't have to necessarily.
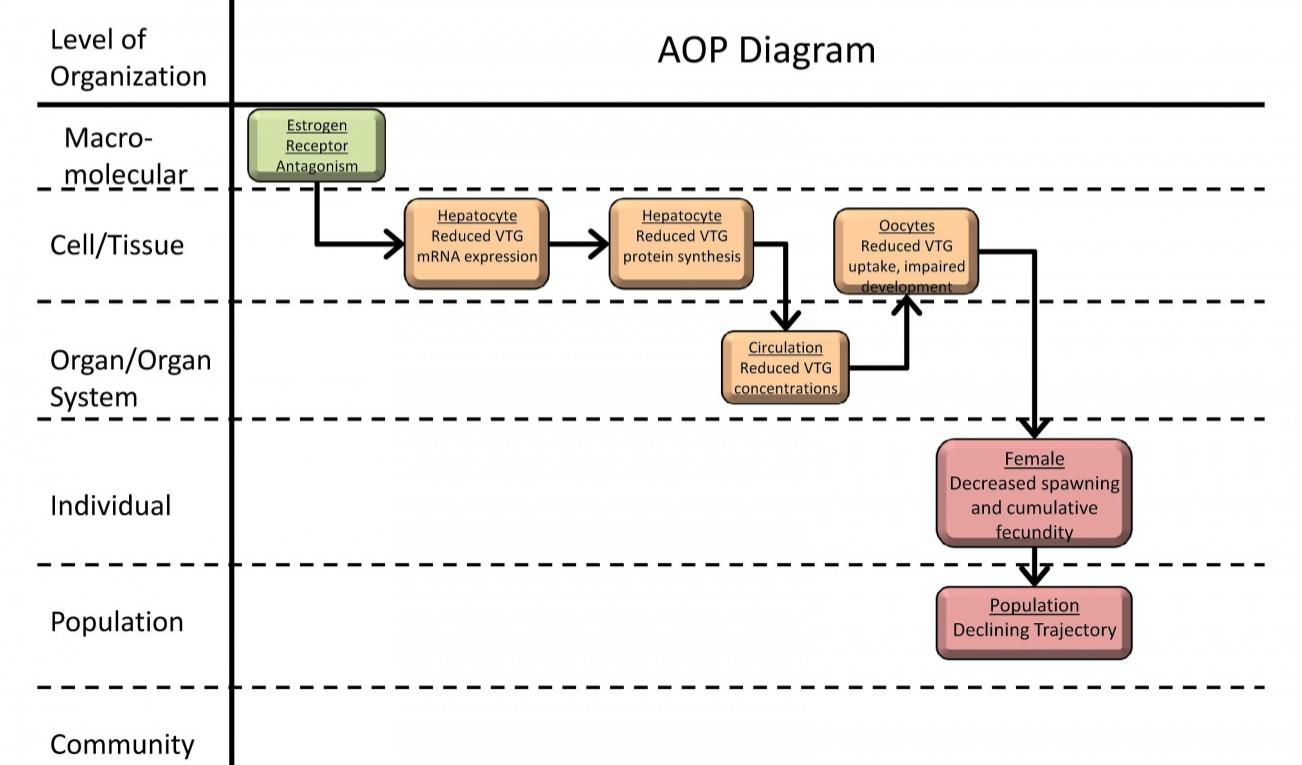
The future of the drug discovery and pharmaceutical product development process and, thus the future of medical cannabis products, lies in the establishment and application of AOPs. (I initiated the concept of "Adverse Outcome Pathways," or AOPs in Part 2 of this series.) The figure below shows a graphical interpretation of an AOP proposing estrogen receptor antagonism as a (mode of action) MOA for reproductive dysfunction in fish.
An example of an AOP from the AOP-Wiki (“Estrogen receptor antagonism leading to reproductive dysfunction”). Reproduced from https://aopkb.org/aopwiki/index.php/Aop:30.
One can quickly visualize how the information is organized is order to document how a “molecular initiating event” -- in this case, antagonism of the estrogen receptor -- starts a cascade of “key events” up through successive layers of biological organization to produce an “adverse outcome” of decreased spawning and fecundity in individual fish. It is important to note that the diagram above was generated from the existence of published literature and other source of information on the known toxico/pharmacokinetics (how a substance gets into the body and what happens to it in the body) and toxico/pharmacodynamics (the molecular, biochemical, and physiological effects of a substance, or its metabolites, within the body).
In the case of cannabinoids, there is a wealth of older published literature that can be mined to provide the same basic schematic diagram. For example, the structure of cannabidiol was first published in 1963 by Mechoulam and Shvo.
(Raphael Mechoulam is an enormously influential figure in this area of science and is still performing research in this area today, for the Hebrew University of Israel. His work was instrumental in elucidating the existence of the endocannabinoid signaling system.)
In addition to the older literature that lays down basic knowledge of molecular mechanism (see some examples here and here and here), there was the establishment of the understanding of the endocannabinoid system in the 1990s, which fostered a more recent age of high-quality studies (see here and here for some examples of review papers). Some of the areas being explored more recently include:
Angiogenesis (the formation of new blood vessels from pre-existing ones, which can control vascular density in cancerous tumors): some cannabinoids that bind to receptors CB1 and CB2 have been shown to inhibit vascular cell survival and migration (Blazquez et al 2003), suppress production of pro-angiogenesis factors (Blazquez et al 2004) and directly induce vascular endothelial cell death (apoptosis).
- Digestive Disorders: a role for CB2 receptors has emerged from research with patients with inflammatory bowel disease and may represent a "braking system" for gut inflammation and its associated symptoms (as reviewed by Wright et al 2008, also see Kunos and Pacher 2004)
- Neuropathic and chronic pain control: While the evidence base for use of phytocannabinoids to control pain is fairly solid, what is lacking is greater knowledge about the molecular targets of cannabinoid-induced analgesic effects seen in both animal models and limited human clinical trials. More recently, glycine receptors have been investigated as potential potentiators of CB-receptor-mediated processes (as reviewed by Xiong et al 2012)
- Antioxidant properties: Those cannabinoids with a phenol group have mild antioxidant properties to protect neurons from oxidative stress (found in both animal models and in vitro studies) and potential molecular mechanisms are discussed by Borges et al 2013.
The process of establishing AOPs not only provides a framework for organizing the existing information for a chemical’s bioactivity but allows one to pinpoint where the data gaps lie in order to focus later research and/or development of new assays or biomarkers of effect.
Does the binding of the CB1 or CB2 receptor by a phytocannabinoid start a forward cascade that leads to an "adverse" outcome? Or it is the accumulation of binding events at the tissue level that leads to an expression of certain proteins that can be measured and this represents a better (or cheaper?) point to measure to understand bioactivity? It is crucial to note that in the application of this thinking to phytocannabinoid drug production that the OUTCOME in this case is not necessarily ADVERSE, it may be a desired health outcome (like reduction of inflammation through the inhibition of pro-inflammatory cytokines) ... so don't too hung up on the word "adverse" in this sense.
What is now sorely needed to be able to truly harmonize international attempts at understanding the molecular mechanisms of phytocannabinoids is a large-scale AOP project similar to the one run by the OECD. I would liken this to a "Manhattan Project" for establishing networks of AOPs that describe in detail the various pathways under consideration for development of phytocannabinoid medicines. Ideally, information generated would be included in existing relevant databases (i.e. the AOP Knowledge Base) and available in open-access form for all researchers to use. This is already the case with some of the available genomic data.
But this isn't all that's needed to optimize the development of custom blends of cannabinoid drugs that are "fit for purpose" (bespoke) based on biological evidence (i.e. known to perturb a certain pathway at a specific dose leading to a predictable and measurable effect). There is knowledge to be gained from the various genomics initiatives underway as well.
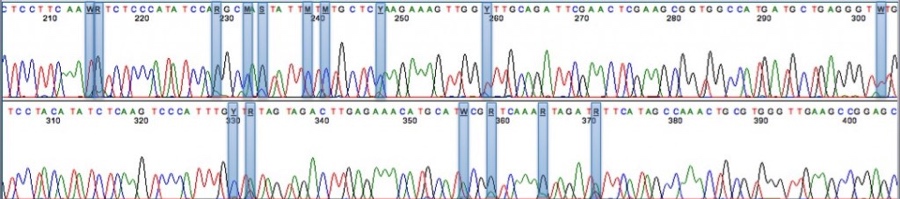
Already, next-generation genomic sequencing technology is being brought to bear to understand the genome of Cannabis sativa and how this can be manipulated to affect strain quality and crop yield (go check out the Medicinal Genomics project!)
This sequencing readout from Medicinal Genomics shows various polymorphisms in the THCA synthase gene, which in part controls genetic variability.
The full genome and transcriptome of Cannabis sativa was published in 2011 and the Cannabis Genome Research Initiative seeks to expand on this work by creating an ultra-high density genetic map, full cultivar history and phylogeny as well as morphologic difference across strains. This information will help optimize strains for production of specific cannabinoids in "cannabis" as well as "hemp" (rich in non-psychoactive CBD but low in psychoactive THC).
Given there are 70-80 phytocannabinoids alone necessitate the use of existing high-throughput modern toxicology approaches. Further given the unknown role that the > 100 terpenoids play along with the known phytocannabinoids only extends that need. Custom combinations of cannabinoids and terpenoids could be studies in the future using knowledge gained through the AOP process in combination with high-throughput screening and molecular and genomic characterization. Current theories regarding the "entourage effect" (great article by another famous researcher, Ethan Russo formerly of GW Pharmaceuticals) could be explored more rapidly given some of the methods discussed above.
In summary, the remarkable advances in technology that have allowed modern toxicology approaches to take root in the pharmaceutical and crop protection industries need to brought to the medical cannabis market. By combining the modern toolbox with the more recent advances in MOA/AOP frameworks, custom “integrated approaches to testing and assessment” (IATA) can be brought to bear that facilitate the collection and organization of data in a way that should save resources and decrease time to the marketplace. It’s time to apply this knowledge to the development of refinement of safe and efficacious medical cannabis & hemp products in a large-scale international research effort: Molecular Mechanisms of Phytocannabinoid Bioactivity.
Back to Part 1 ...
How recent advances in toxicology will revolutionize the medical cannabis industry: Part 3
There are two major revolutions unfolding whose paths are about to cross … not only for the betterment of the cannabis and hemp industries but for the future of toxicology as well.
Part 3: The Status of Medicinal Cannabis
http://cdn3.collective-evolution.com/assets/uploads/2014/01/cann.jpeg
Medicinal cannabis programs have now been established in the vast majority of states (38 so far), starting in 1996 with Prop 215/Compassionate Use Act in California -- where, curiously enough, this 20-year old program only recently overhauled its regulatory programs to ensure a greater degree of transparency and increase accountability (The Governor signed off on the changes just last week). According to NORML, the following states have yet to adopt any sort of medicinal cannabis program: Idaho, Texas, Kansas, Nebraska, North and South Dakota, Louisiana, West Virginia, Arkansas, Indiana, Ohio and Pennsylvania. Although it should be noted that these states are not closed to the issue and, in fact, all of them (with the exception of Idaho and Louisiana) have either approved or are considering legislation to establish industrial hemp programs (from which medicinal products can be derived like "hemp oil").
Of course, every state program is different per its individual legislative mandates and operating conditions. There are now many states that have separately approved the use of cannabidiol (CBD) oils for medical treatments: Utah, Wyoming, Oklahoma, Iowa, Missouri, Wisconsin, Kentucky, Virginia, Tennesee, North and South Carolina,, Mississippi, Alabama, Georgia and Florida. It should be noted that other seemingly unconnected laws also impact the individual nature of each state's program ... such as banking and finance laws, product liability laws, and pre-emption/local control laws (not to mention how seemingly common terms like "institution of higher learning" may be defined differently by different state legislative code). It is therefore, incredibly important, to deeply dive into your state's operating conditions (defined by both legislative mandates, state agency policies, and market and consumer needs) prior to joining the industry.
http://globenewswire.com/news-release/2013/12/03/594185/10060136/en/Medical-Marijuana-Inc-s-HempMedsPX-Represents-High-Times-Cannabis-Cup-Highest-CBD-Concentrate-Award-Winner.html
Medicinal cannabis (in all its forms) has a variety of different therapeutic uses, some with solid evidence bases and some without. However, every state has a different list of "treatable conditions," which include several ailments for which there is not a solid evidence base. The largest medical dispensary in the world, Harborside Health Center in northern California, offers a web page listing the known treatable illnesses and popular information service Leafly lists a state-by-state compendium of qualifying conditions. Some states only low-THC, CBD-enriched products to treat a narrow window of disorders or conditions, while other states have fairly monstrous lists. Not to pick on Illinois, but it has the most sprawling list of treatable conditions and it would be hard to find solid evidence to support all these conditions (yet):
Qualifying conditions to become a medical marijuana patient in Illinois include:
- Acquired Immunodeficiency Syndrome (AIDS)
- Alzheimer's disease
- Autism
- Lou Gehrig's disease (ALS)
- Arnold-Chiari malformation and syringomyelia
- Cachexia/wasting syndrome
- Cancer
- Causalgia
- Chronic inflammatory demyelinating polyneuropathy
- Chronic post-operative pain
- Chronic pain due to trauma
- Chronic Pain Syndrome
- Crohn's disease
- CRPS (Complex Regional Pain Syndrome Type I)
- CRPS (Complex Regional Pain Syndrome Type II)
- Dystonia
- Fibromyalgia (severe)
- Fibrous dysplasia
- Glaucoma
- Hepatitis C
- Human Immunodeficiency Virus (HIV)
- Hydrocephalus
- Interstitial cystitis
- Intractable pain
- Irritable bowel syndrome
- Lupus
- Multiple sclerosis
- Muscular dystrophy
- Myasthenia gravis
- Myoclonus
- Nail-patella syndrome
- Neurofibromatosis
- Osteoarthritis
- Parkinson's disease
- Post-concussion syndrome
- Post-traumatic stress disorder (PTSD)
- Residual limb pain
- Rheumatoid arthritis (RA)
- Seizures
- Sjogren's syndrome
- Spinal cord disease (including but not limited to arachnoiditis, Tarlov cysts, hydromyelia & syringomelia)
- Spinal cord injury
- Spinocerebellar ataxia (SCA)
- Tourette syndrome
- Traumatic brain injury (TBI)
The evidence base for therapeutic use of cannabis was reviewed most recently by Kevin Hill in JAMA, who reviewed the literature from 1948-2015 and focused on 28 randomized clinical trials using cannabinoids for conditions other than those approved by FDA (see below for info on FDA-approved cannabis products). He found that high-quality evidence supports the use of cannabis for chronic pain, nerve pain (neuropathy), and muscle spasms/spasticity due to MS or paraplegia. In fact, the greatest interest might be the potential for cannabinoid pain medications to replace or complement opioid-based pain medications as the cannabinoids have been suggested to have an improved “therapeutic window” relative to opioids for pain control (Hayes and Brown 2014; JAMA Intern Med). There's little question that cannabinoid therapy for chronic pain control poses a much lower potential for abuse than opioid-based therapies and absolutely no question that cannabis poses much lower acute toxicity! According to a June 2015 report by the Pew Charitable Trust, about 50% of deaths due to drug overdose (22,000 per year) are related to prescription drugs, including legally prescribed opioid narcotics.
The differences in treatable conditions across the states in relation to the amount of evidence supporting each type of therapy can lead to some challenging risk communication between doctor and patient. In addition, patients get knowledge on the topic from social media sites and other poor-quality information sources. For example, a recent meme on Facebook promised medicinal cannabis provided "34 cures for cancer," which appears to have been supported from an article listing 34 studies "proving cannabis cures cancer." This is not to say there have not been some interesting discoveries in the world of cancer research; however, we are a long way off from being able to efficaciously treat tumors with cannabinoid therapy. It must be noted that the collective ability of the scientific community to develop an evidence base is restricted by the current Schedule 1 status as dictated by the Controlled Substance Act of 1970 (although there is a measure currently in the Senate to change the classification of cannabis to Schedule 2, which would allow FDA, NIH etc. to fund larger research efforts).
One application of great interest is the use of CBD oils to treat epilepsy in children; however, the promise of these applications found on social media, etc. have so far outpaced the medical community’s ability to provide a solid evidence base. CBD oils hold much promise for the treatment of "intractable" (severe) forms of epilepsy, such as Dravet and Lennox-Gastaut syndromes. However, like other forms of medicinal cannabis, detailed knowledge of toxicological mechanism and efficacy is still developing ... there are ongoing clinical trials for CBD oils in the treatment of severe epilepsy and many tech/pharma companies are out there competing in the marketplace.
What we lack though is knowledge on the mechanism of toxicity as well as a completed mode of action framework (such as an Adverse Outcome Pathways, which I have described elsewhere) that shows the trigger points for bioactivity from the molecular level all the way up to the individual human level. A September 2015 publication in the New England Journal of Medicine reviewed the evidence for treatment of epilepsy, which I have described elsewhere. The conclusions of Friedman and Devinsky (2015) are that preclinical animal and in vitro data as well as some preliminary human data “may be effective in treatment with some patients with epilepsy” but “current data from studies in humans are extremely limited and no conclusions can be drawn.”
There are several FDA-approved medicinal cannabis products on the market today that should be mentioned. The synthetic cannabinoid drugs dronabinol (Marinol® from AbbVie) and nabilone (Cesamet® from Meda Pharmaceuticals) are used to treat nausea and vomiting due to chemotherapy; former also used to treat loss of appetite and “wasting” in AIDS/cancer patients. As of 2013, FDA was in clinical trial phase 3 with nabiximols (Sativex ® from GW Pharmaceuticals), a vaporized inhalant alternative to oral treatments currently available to treat chronic pain from cancer treatment (See Bostwick et al 2013; NEJM) which has now been approved in 27 other countries for treatment of spasms. In addition, the American Academy of Neurology published evidence-based guidelines in 2014 for the treatment of selected neurological disorders (including spasticity).
Currently, there are 273 clinical trials (of all type: ongoing, planned, withdrawn or complete) ongoing in US alone for the use of Sativex for a number of disorders. California, New York, Maryland and Massachusetts are associated with almost 60% of all US Sativex clinical trials. One particularly noteworthy study to examine "behavioral pharmacology and toxicology of oral cannabis" is currently recruiting patients at my alma mater, Johns Hopkins University.
Data and presentation from clinicaltrials.gov
It is important to note that these data are imprecise and the reader is encouraged to search at http://clinicaltrials.gov for more detailed information. For example, the aforementioned Johns Hopkins study will provide crucial human kinetics data that can help establish an adverse outcome pathway to allow for precise identification of biomarkers for clinical, workplace, regulatory and law enforcement needs.
But I will discuss this concept and more in Part 4: Two Roads Converged in the Woods ...
Follow-up on the case of the Potomac "green goo"
Recently, the state of Maryland released this information on the MDE web site on the spill of latex into the Potomac River:
The Maryland Department of the Environment received reports on September 24, 2015, of a yellow/white coloration in the North Branch Potomac River. MDE sent an investigator to the site and the Department also contacted the Verso paper mill and the Upper Potomac River Commission plant that treats wastewater from the paper mill. The paper mill said the mill had an approximately 10,000 gallon spill of latex, which is used for paper coating, over a four-hour period after off-loading of a rail car and that the spill was discharged through their collection system to the Upper Potomac River Commission plant.
According to information provided by the Verso paper mill to MDE, the substance that was discharged is Latex CP 620NA, manufactured by Trinseo LLC.
According to information from the manufacturer’s Material Safety Data Sheet:
The product is not a hazardous chemical as defined under U.S. Occupational Safety and Health Administration regulations.
The components of the material are styrene-butadiene based polymer and water.
Because the substance is in the water column of a flowing river, it is not possible to contain the spill or to remove it from the water. It is expected that the substance will continue to flow down the river and become diluted.
In keeping with the Department’s practice for spills and discharges, MDE notified the Interstate Commission on the Potomac River Basin for notification to drinking water facilities not only in Maryland but in Virginia, West Virginia and Washington, D.C.
MDE’s primary focus at this time is to ensure that public health and the environment are protected. Information available to MDE at this time does not indicate a health concern. MDE continues to work to obtain additional information, including laboratory analysis of water samples collected from affected locations of the river.
Laboratory results received to date have shown no detection of styrene, the primary constituent of concern, and no evidence of butadiene, another constituent of concern. MDE has provided the laboratory testing results to the Interstate Commission on the Potomac River Basin. MDE will continue to provide information to the Interstate Commission on the Potomac River Basin and drinking water systems.
Sample results have been posted as well. Samples were collected at three different time points at three different locations (so understanding any temporal or spatial trends in the data will not be possible) ... on Sept 25, 26 and 29 at Pinto (Maryland), Route 28 bridge crossing and Bonds Landing, respectively.
It appears that only a single sample was taken each day from each location and these were analyzed using standard EPA analytical methods for a wide number of volatiles, semivolatiles, metals and standard water chemistry parameters. It appears to have been a tag-team effort between Maryland Department of the Environment and the EPA Region 3 in terms of the sample collection (and it should be noted from a regulatory geek perspective, only the MDE-collected samples has "chain of custody" paperwork associated with it...)
Data summary from the MDE page focuses on styrene and 1,3-butadiene, which are the major building blocks of the latex polymer that spilled into the river. It is good to verify the concentrations of these; however, a quick review of available data on environmental fate & transport reveals that much of the volatile component of the mixture would off-gas from the water as it traveled down the river channel. What wasn't done was to look at the potential for degradants that form in the water column once they come into contact with any chemical constituents. But the polymer wasn't expected to degrade signficantly according to the MSDS sheet.
Even though the potential danger has now passed and was a fairly low-level hazard/threat to begin with, this incident points out some obvious gaps in the response and communication process across states. Even though Maryland and West Virginia share the same source of drinking water (Potomac River), it appears as if Maryland didn't feel alerting the neighbors on the other side of the water was a very high priority. I was passed back and forth between county and state in trying to get answers from the state of Maryland in the days following the release. The state of West Virginia never bothered to respond, once the country referred me to the state. In fact, it took multiple attempts after the fact to get the public web site info listed above from Maryland. Similarly, the Interstate Commission on the Potomac River Basin didn't respond to my attempts to get information even though it clearly had been given the testing results (as claimed by the state of Maryland).
Who loses in this situation? THE CONSUMER. The END USER. We *deserve* the information. Governments on all level who supply or protect drinking water should be more forthcoming with information to its constituents following emergencies like this. Despite the physiographic differences among them.
How recent advances in toxicology will revolutionize the medical cannabis industry: Part 2
There are two major revolutions unfolding whose paths are about to cross … not only for the betterment of the cannabis and hemp industries but for the future of toxicology as well.
Part 2: The Future of Toxicology
While the study of poisons (toxicology) and drugs (pharmacology) have been occurring for thousands of years, the “father” of modern toxicology is often identified as Paracelsus, who stated in 1493:
“All substances are poisons; there is none which is not a poison. The right dose differentiates a poison from a remedy.”
Many classical discoveries in the field were made in the first half of the 20th century (chemical weapons, the psychedelic drug LSD, vitamin C, and the popular insecticide DDT to name but a few); however, it wasn’t until 1975 that the field had its first textbook published. (Louis J. Casarett and John Doull edited Toxicology: The Basic Science of Poisons). For centuries now, conclusions on the adverse health effects from toxicological experiments have been primarily drawn from whole-animal studies where “apical” (effects seen at the whole organism level, like weight loss) and organ-level findings (often assessed through biochemical means or through histopathological examination) are observed and associated with the dose given to the animal (so-called conventional toxicology).
This started to change with advent of in vitro techniques in the late 1970s and the explosion of information from molecular and cellular biology from the 1980s onward (what I refer to as modern toxicology).
Today, there’s no question that the field of toxicology is currently undergoing a paradigm shift that will modify the way that “conventional” testing approaches are utilized to demonstrate safety profiles for chemicals and drugs in the marketplace. This paradigm shift has been made possible by technical advances in in bioinformatics, molecular & systems biology, toxicogenomics and computational & cellular toxicology and “envisioned” by a seminal 2007 report from the National Research Council. This shift is moving the focus towards understanding “pathways of toxicity” by characterizing a chemical’s mode-of-action (MOA) through the identification of key events at the molecular, cellular, and tissue/organ level – perturbation of which leads to some undesired, “adverse” outcome. (You can read a previous piece I have written about MOA frameworks for organizing information on toxicity pathways here.)
Reproduced from the 2007 NAS report, "Toxicity Testing in the 21st Century." The central aspect of this new vision is elucidation of toxicity pathways from the molecular level up, followed by "ground truthing" in human populations using biomarkers or molecular epidemiology studies.
This modern approach is very different from the current approach to toxicity testing, which relies on identifying observable effects through a complex assembly of study types using whole animals, where some change in pathology or clinical indicator is assumed to be representative of some undesired, “adverse” state (i.e. as NRC says, “indicative of a disease state”). As such, it is necessary to capture MOA data in a clear fashion that is amenable to the identification of key events that can be then tested empirically (using existing or yet-to-be-developed methods) to demonstrate the quantitative relationship between said events and adverse outcome and eventually, develop predictive toxicology models. This approach has been taken for nanomaterial characterization as well as for understanding the effects of "endocrine disruptors" on human health & the environment.
The future lies in the application of these MOA frameworks – such as the Adverse Outcome Pathway (AOP) approach - to the development of new drugs and chemicals. The development of detailed AOPs to describe the key events in a particular toxicity pathway holds enormous promise to not only gaining a deeper understanding into a chemical's physiological mechanism of action but to help design "intelligent" and "integrated approaches to testing and assessment" (IATAs). A very recent success story in the use of AOPs can be found in Europe, where a guidance document for an IATA for skin irritation and corrosion has just been published to replace the existing animal-based toxicity test for dermal irritation and corrosion.
The international collaboration that is currently unfolding in the development of AOPs needs to now incorporate the phytocannabinoids of greatest interest to the medicinal cannabis field.
http://www.medicinalgenomics.com/wp-content/uploads/2011/09/phytocannabinoid-terpenoid_2.png
How recent advances in toxicology will revolutionize the medical cannabis industry: Part 1
There are two major revolutions unfolding whose paths are about to cross … not only for the betterment of the cannabis and hemp industries but for the future of toxicology as well.
This multi-part article will describe these revolutions and how they will intersect.
Part 1: Introduction
Gene expression analysis data and the cannabinoid biosynthesis pathway. Reproduced from van Bakel et al. (2011) in Genome Biology.
“In this world there are only two tragedies. One is not getting what one wants and the other is getting it.”
After several thousands of years of cultivation as a medicinal plant, the proponents of medical cannabis have finally “gotten what one wants” in the form of state-sanctioned production and regulatory programs. Now what? There are a handful of cannabinoid-based products on the market that have been approved by the US Food & Drug Administration, each with its own sets of pros and cons and all developed without the incredible toolbox that has been developed in recent years to understand and measure how chemicals interact with biological systems on a molecular basis.
Today, we stand at the convergent crossroads of an enormous legislative shift (i.e. the liberalization of cannabis laws and the resultant rapid expansion of a new agricultural, drug and consumer product industry) and an equally enormous paradigmatic shift in the fields of toxicology and pharmacology. Our increased ability to understand how chemicals lead to biological activity from a “mode of action” perspective (from experiments such as the gene expression data shown above) can be harnessed to establish the next generation of cannabis-based drugs, drawing on the ongoing interdisciplinary efforts of the toxicology, computational biology, bioinformatics and analytical testing communities. (I will describe "mode of action" and its importance in Part 2.)
https://www.flickr.com/photos/bengalsfan1973/2854305903
By understanding on a more refined level how cannabinoid-based drugs lead to biological activity via "pathways of toxicity" from cells to organ systems, the advanced techniques and approaches used in pharmacology & toxicology (and by many pharmaceutical and chemical companies) can be brought to the development of cannabinoid drugs. This will revolutionize the medical cannabis industry in a way that reduces animal use, leads to drugs with fewer side effects, leads to more efficacious formulations and could simplify introductions of products to the market by avoiding the large expensive and longer timelines to approval associated with long-term animal tests. It will also provide insight into the development of future assays to demonstrate product safety in the non-medical marketplace.
Next ... Part 2: The Future of Toxicology.
What is with that "green goo" in the Potomac River?
On September 23, at the Verso Company paper mill on the N. Branch of the Potomac River (near Luke, Maryland), just under 10,000 gallons of "latex" was released during a four-hour period as it was being unloaded from a rail car. A "Verso worker failed to close a drain line on a 26,500-gallon storage tank that was being filled from a railroad tank car at the mill,"
According to a piece in the Cumberland Times-News from September 25, the spill was "discharged through a collection system." Attempts to clarify how much of the release was retained by the collection system were deferred by Verso to the Upper Potomac River Commission. The Luke plant receives latex material via rail car to produce specialty paper that has a latex-based coating. (Latex binds the coating to the paper and imparts high-quality optical properties.)
An example of Verso labeling paper, reproduced from the company website.
Over the weekend, a plume of "green goo" was seen in the Potomac near Cumberland. On Monday (today), the Times-News reported that Paw Paw, WV shut its water intake system (to provide drinking water to the town from the river) to avoid "gumming up the works" literally. It is anticipated to reach the Hagerstown water intake by this Saturday (October 4).
The product that spilled was an emulsion (or mixture) of water and a "styrene-butadiene based polymer" referred to as "Latex CP 620NA" and manufactured by Trinseo LLC.
A "safety data sheet" dated 2013 was procured from the company (available upon request) and it provides the following information:
- "may cause temporary eye irritation" and "prolonged contact may cause slight skin irritation;" however, "prolonged skin contact is unlikely to result in absorption of harmful amounts"
- the substance generally poses very low toxicity via oral and dermal exposure; "practically nontoxic to aquatic organisms on an acute basis"
- coagulation of the material can occur in low temperatures and with the "addition of chemicals, such as acids or multivalent salts"
- decomposition of the material in the environment depends on the ambient temperature, the supply of oxygen from air and the presence of other materials; however "the polymeric component is not expected to degrade"
The available data on the product seems to be indicate it is fairly non-toxic on an acute basis (upon short-term exposure) to both humans and aquatic life. There appears to little to no data on effects from chronic (repeat) exposure. There is also little reason to worry about chronic exposure for us humans getting our drinking water out of the Potomac as localities have the ability to store clean water and close the water intakes for up to a few days, depending on storage capability.
There is also little information on the types of degradation products that could be expected in the Potomac River water as the slug of pollution makes its way from Allegheny County downstream to the Chesapeake Bay.
No fish kills have been noted as of yet and samples have been collected; however no results have been shared yet with the public. It has been reported that volatile organic chemicals were being sampled for; however, it should be verified that styrene , butadiene and their primary aquatic breakdown products are on that list. Some of these might be classified as semi-volatile and covered by a different testing method.
Some useful information on styrene has been previously published by the CDC: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=421&tid=74
please don't hesitate to contact M³ for additional information and advice.
AOPs are crucial to the ongoing paradigm shift in toxicology
There’s no question that the field of toxicology is currently undergoing a paradigm shift that will modify the way that “conventional” testing approaches are utilized to demonstrate safety profiles for chemicals and finished goods in the marketplace. This paradigm shift has been made possible by technical advances in in bioinformatics, molecular & systems biology, toxicogenomics and computational & cellular toxicology and “envisioned” by the seminal 2007 report from the National Research Council (NRC 2007). This shift is moving the focus towards understanding “pathways of toxicity” by characterizing a chemical’s mode-of-action (MOA) through the identification of key events at the molecular, cellular, and tissue/organ level – perturbation of which leads to some undesired, “adverse” outcome. This modern approach is very different from the current approach to toxicity testing, which relies on identifying observable effects through a complex assembly of study types using whole animals, where some change in pathology or clinical indicator is assumed to be representative of some undesired, “adverse” state (i.e. as NRC says, “indicative of a disease state”). As such, it is necessary to capture MOA data in a clear fashion that is amenable to the identification of key events that can be then tested empirically (using existing or yet-to-be-developed methods) to demonstrate the quantitative relationship between said events and adverse outcome and eventually, develop predictive toxicology models. Such efforts are referred to as MOA frameworks.
Examples of MOA frameworks found in the literature include the MOA/Human Relevance Framework (Meek et al 2003; Boobis et al 2006; Boobis et al 2008), developed jointly by International Program on Chemical Safety and the International Life Sciences Institute, the Key Event Dose-Response Framework (also developed by ILSI) [Julien et al (2009)], “Pathways of Toxicity” as described by Kleensang et al (2014), and the Adverse Outcome Pathway approach as described by Ankley et al (2010) and widely embraced in Europe by the OECD. (See Figure 1 for an easy visualization of how they compare.) It is this latter approach that appears to have the greatest potential for internationally harmonized use, as evidenced by the young, but slowly growing, AOP-Knowledgebase (https://aopkb.org/).
Figure 1. Graphical depiction of the various available MOA frameworks. Reproduced from AltTox.org (http://alttox.org/mapp/emerging-technologies/pathway-based-toxicology/)
The AOP-KB is actually an information hub that not only includes a repository for AOPs (AOP-Wiki) and a graphical interface to explore them (AOP Explorer). Also under development is a tool to use quantitative AOPs (Effectopedia) as well as “intermediate effects” database that promises to place AOP information in a regulatory context. At current moment, only the AOP-Wiki is operational, where both completed, in progress and draft AOPs awaiting public comment are stored. An example of an AOP for estrogen receptor antagonism is shown in Figure 2.
OECD became the primary driver for the development of the AOP-KB in 2012, when the program was announced. In order to add information to the AOP-Wiki, a proposal is sent to the OECD Secretariat and these proposals are then vetted and managed by the European Advisory Group on Molecular Screening and Toxicogenomics in coordination with the IPCS Mode of Action workgroup activity. Proposals are reviewed and accepted twice a year and collaborations across industry, academia and government are encouraged. (See http://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm for more information on submitting AOP proposals, including a flow chart and guidance documentation.)
Figure 2. An example of an AOP from the AOP-Wiki (“Estrogen receptor antagonism leading to reproductive dysfunction”). Reproduced from https://aopkb.org/aopwiki/index.php/Aop:30.
In order to populate AOP-Wiki with a sufficient amount of pathways and data in order to enable its functionality as an international research and analysis tool, it is imperative that practitioners get involved in collaborative projects. In essence, this a clarion call for members of the various scientific disciplines to “roll up their sleeves” and get involved in establishing AOPs in their field of expertise.
Citations:
Ankley, G. T., Bennett, R. S., Erickson, R. J., Hoff, D. J., Hornung, M. W., Johnson, R. D., and Villeneuve, D. L. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry, 29(3), 730–741, 2010.
Boobis, A.R., Cohen, S.M., Dellarco, V., McGregor, D., Meek, M.E., Vickers, C., Willcocks, D., Farland, W. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 36(10):781-92, 2006.
Boobis, A.R., Doe, J.E., Heinrich-Hirsch, B., Meek, M.E., Munn, S., Ruchirawat, M., Schlatter, J., Seed, J., Vickers, C. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38(2):87-96, 2008.
Julien, E., Boobis, A. R., & Olin, S. S. The Key Events Dose-Response Framework: a cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Critical Reviews in Food Science and Nutrition, 49(8), 682–689, 2009.
Kleensang, A. et al. t4 workshop report: Pathways of Toxicity. ALTEX. 31(1):53-61, 2014.
Meek, M.E., Bucher, J.R., Cohen, S.M., Dellarco, V., Hill, R.N., Lehman-McKeeman, L.D., Longfellow, D.G., Pastoor, T., Seed, J., Patton, D.E. A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol. 33(6):591-653, 2003.
National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press, 2007.