There’s no question that the field of toxicology is currently undergoing a paradigm shift that will modify the way that “conventional” testing approaches are utilized to demonstrate safety profiles for chemicals and finished goods in the marketplace. This paradigm shift has been made possible by technical advances in in bioinformatics, molecular & systems biology, toxicogenomics and computational & cellular toxicology and “envisioned” by the seminal 2007 report from the National Research Council (NRC 2007). This shift is moving the focus towards understanding “pathways of toxicity” by characterizing a chemical’s mode-of-action (MOA) through the identification of key events at the molecular, cellular, and tissue/organ level – perturbation of which leads to some undesired, “adverse” outcome. This modern approach is very different from the current approach to toxicity testing, which relies on identifying observable effects through a complex assembly of study types using whole animals, where some change in pathology or clinical indicator is assumed to be representative of some undesired, “adverse” state (i.e. as NRC says, “indicative of a disease state”). As such, it is necessary to capture MOA data in a clear fashion that is amenable to the identification of key events that can be then tested empirically (using existing or yet-to-be-developed methods) to demonstrate the quantitative relationship between said events and adverse outcome and eventually, develop predictive toxicology models. Such efforts are referred to as MOA frameworks.
Examples of MOA frameworks found in the literature include the MOA/Human Relevance Framework (Meek et al 2003; Boobis et al 2006; Boobis et al 2008), developed jointly by International Program on Chemical Safety and the International Life Sciences Institute, the Key Event Dose-Response Framework (also developed by ILSI) [Julien et al (2009)], “Pathways of Toxicity” as described by Kleensang et al (2014), and the Adverse Outcome Pathway approach as described by Ankley et al (2010) and widely embraced in Europe by the OECD. (See Figure 1 for an easy visualization of how they compare.) It is this latter approach that appears to have the greatest potential for internationally harmonized use, as evidenced by the young, but slowly growing, AOP-Knowledgebase (https://aopkb.org/).
Figure 1. Graphical depiction of the various available MOA frameworks. Reproduced from AltTox.org (http://alttox.org/mapp/emerging-technologies/pathway-based-toxicology/)
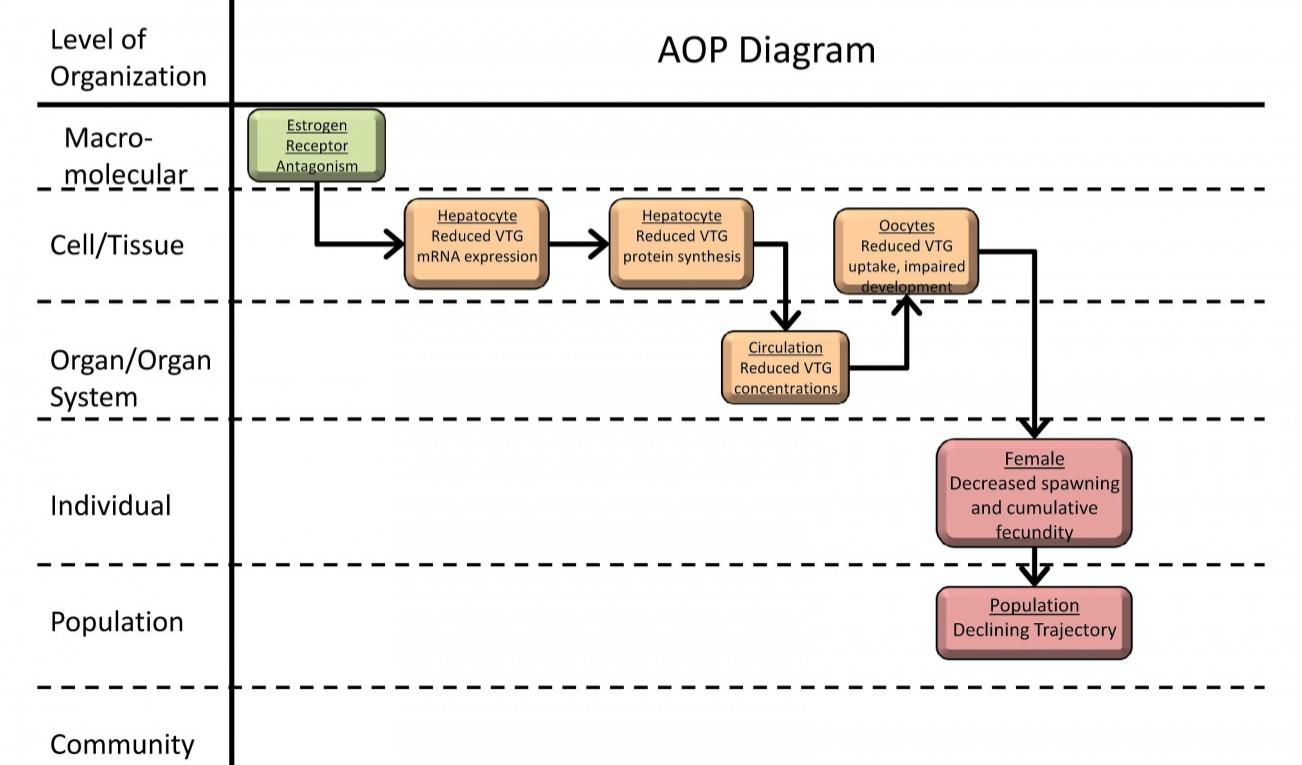
The AOP-KB is actually an information hub that not only includes a repository for AOPs (AOP-Wiki) and a graphical interface to explore them (AOP Explorer). Also under development is a tool to use quantitative AOPs (Effectopedia) as well as “intermediate effects” database that promises to place AOP information in a regulatory context. At current moment, only the AOP-Wiki is operational, where both completed, in progress and draft AOPs awaiting public comment are stored. An example of an AOP for estrogen receptor antagonism is shown in Figure 2.
OECD became the primary driver for the development of the AOP-KB in 2012, when the program was announced. In order to add information to the AOP-Wiki, a proposal is sent to the OECD Secretariat and these proposals are then vetted and managed by the European Advisory Group on Molecular Screening and Toxicogenomics in coordination with the IPCS Mode of Action workgroup activity. Proposals are reviewed and accepted twice a year and collaborations across industry, academia and government are encouraged. (See http://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm for more information on submitting AOP proposals, including a flow chart and guidance documentation.)
Figure 2. An example of an AOP from the AOP-Wiki (“Estrogen receptor antagonism leading to reproductive dysfunction”). Reproduced from https://aopkb.org/aopwiki/index.php/Aop:30.
In order to populate AOP-Wiki with a sufficient amount of pathways and data in order to enable its functionality as an international research and analysis tool, it is imperative that practitioners get involved in collaborative projects. In essence, this a clarion call for members of the various scientific disciplines to “roll up their sleeves” and get involved in establishing AOPs in their field of expertise.
Citations:
Ankley, G. T., Bennett, R. S., Erickson, R. J., Hoff, D. J., Hornung, M. W., Johnson, R. D., and Villeneuve, D. L. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry, 29(3), 730–741, 2010.
Boobis, A.R., Cohen, S.M., Dellarco, V., McGregor, D., Meek, M.E., Vickers, C., Willcocks, D., Farland, W. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 36(10):781-92, 2006.
Boobis, A.R., Doe, J.E., Heinrich-Hirsch, B., Meek, M.E., Munn, S., Ruchirawat, M., Schlatter, J., Seed, J., Vickers, C. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38(2):87-96, 2008.
Julien, E., Boobis, A. R., & Olin, S. S. The Key Events Dose-Response Framework: a cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Critical Reviews in Food Science and Nutrition, 49(8), 682–689, 2009.
Kleensang, A. et al. t4 workshop report: Pathways of Toxicity. ALTEX. 31(1):53-61, 2014.
Meek, M.E., Bucher, J.R., Cohen, S.M., Dellarco, V., Hill, R.N., Lehman-McKeeman, L.D., Longfellow, D.G., Pastoor, T., Seed, J., Patton, D.E. A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol. 33(6):591-653, 2003.
National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press, 2007.