There are two major revolutions unfolding whose paths are about to cross … not only for the betterment of the cannabis and hemp industries but for the future of toxicology as well.
Part 2: The Future of Toxicology
While the study of poisons (toxicology) and drugs (pharmacology) have been occurring for thousands of years, the “father” of modern toxicology is often identified as Paracelsus, who stated in 1493:
“All substances are poisons; there is none which is not a poison. The right dose differentiates a poison from a remedy.”
Many classical discoveries in the field were made in the first half of the 20th century (chemical weapons, the psychedelic drug LSD, vitamin C, and the popular insecticide DDT to name but a few); however, it wasn’t until 1975 that the field had its first textbook published. (Louis J. Casarett and John Doull edited Toxicology: The Basic Science of Poisons). For centuries now, conclusions on the adverse health effects from toxicological experiments have been primarily drawn from whole-animal studies where “apical” (effects seen at the whole organism level, like weight loss) and organ-level findings (often assessed through biochemical means or through histopathological examination) are observed and associated with the dose given to the animal (so-called conventional toxicology).
This started to change with advent of in vitro techniques in the late 1970s and the explosion of information from molecular and cellular biology from the 1980s onward (what I refer to as modern toxicology).
Today, there’s no question that the field of toxicology is currently undergoing a paradigm shift that will modify the way that “conventional” testing approaches are utilized to demonstrate safety profiles for chemicals and drugs in the marketplace. This paradigm shift has been made possible by technical advances in in bioinformatics, molecular & systems biology, toxicogenomics and computational & cellular toxicology and “envisioned” by a seminal 2007 report from the National Research Council. This shift is moving the focus towards understanding “pathways of toxicity” by characterizing a chemical’s mode-of-action (MOA) through the identification of key events at the molecular, cellular, and tissue/organ level – perturbation of which leads to some undesired, “adverse” outcome. (You can read a previous piece I have written about MOA frameworks for organizing information on toxicity pathways here.)
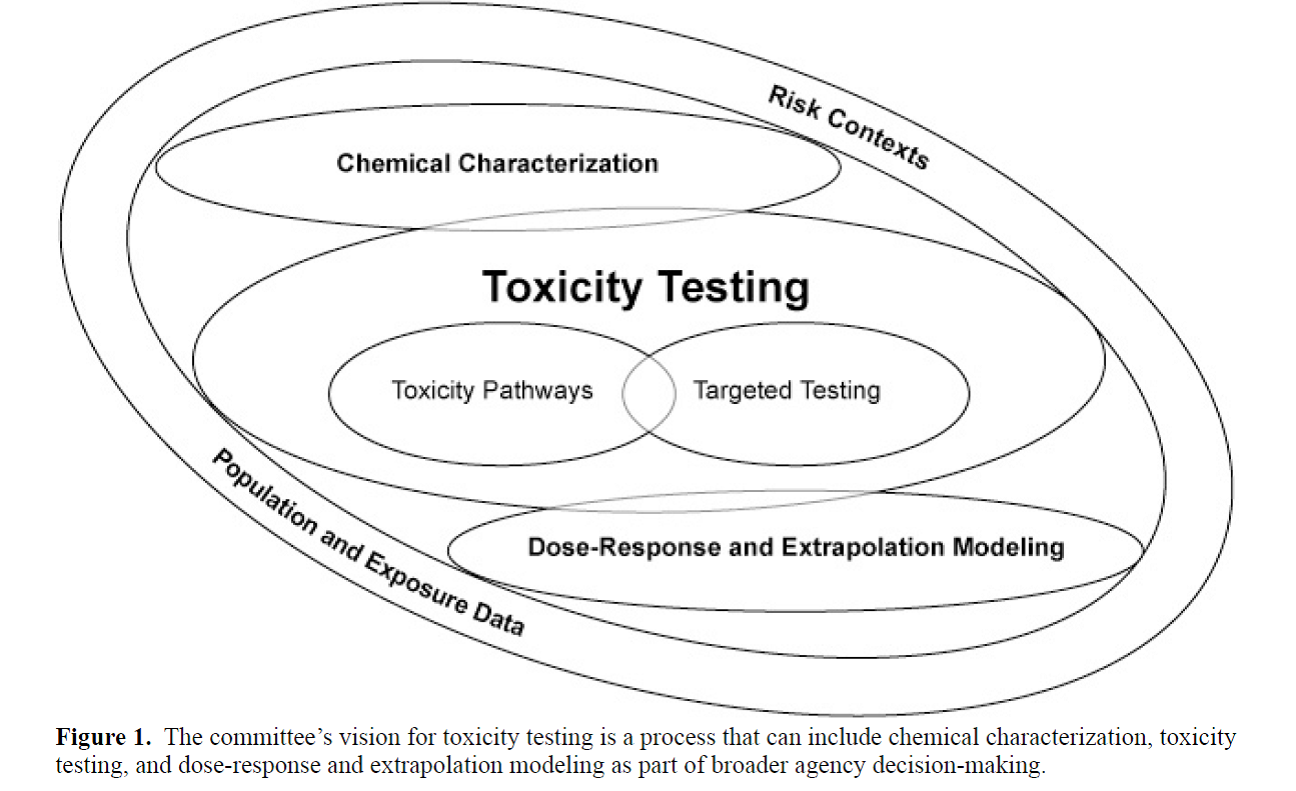
Reproduced from the 2007 NAS report, "Toxicity Testing in the 21st Century." The central aspect of this new vision is elucidation of toxicity pathways from the molecular level up, followed by "ground truthing" in human populations using biomarkers or molecular epidemiology studies.
This modern approach is very different from the current approach to toxicity testing, which relies on identifying observable effects through a complex assembly of study types using whole animals, where some change in pathology or clinical indicator is assumed to be representative of some undesired, “adverse” state (i.e. as NRC says, “indicative of a disease state”). As such, it is necessary to capture MOA data in a clear fashion that is amenable to the identification of key events that can be then tested empirically (using existing or yet-to-be-developed methods) to demonstrate the quantitative relationship between said events and adverse outcome and eventually, develop predictive toxicology models. This approach has been taken for nanomaterial characterization as well as for understanding the effects of "endocrine disruptors" on human health & the environment.
The future lies in the application of these MOA frameworks – such as the Adverse Outcome Pathway (AOP) approach - to the development of new drugs and chemicals. The development of detailed AOPs to describe the key events in a particular toxicity pathway holds enormous promise to not only gaining a deeper understanding into a chemical's physiological mechanism of action but to help design "intelligent" and "integrated approaches to testing and assessment" (IATAs). A very recent success story in the use of AOPs can be found in Europe, where a guidance document for an IATA for skin irritation and corrosion has just been published to replace the existing animal-based toxicity test for dermal irritation and corrosion.
The international collaboration that is currently unfolding in the development of AOPs needs to now incorporate the phytocannabinoids of greatest interest to the medicinal cannabis field.
http://www.medicinalgenomics.com/wp-content/uploads/2011/09/phytocannabinoid-terpenoid_2.png