There are two major revolutions unfolding whose paths are about to cross … not only for the betterment of the cannabis and hemp industries but for the future of toxicology as well.
Part 4 : Two Roads Converged in the Woods ... ?
OK, perhaps it's not the woods. And this is not the classic poem by Robert Frost about roads diverging. But that's because we are not "out of the woods" yet when it comes to unlocking the molecular mode-of-action for the collection of conditions and diseases that phytocannabinoids may be able to treat. And the path converges far ahead down the road ... but it doesn't have to necessarily.
The future of the drug discovery and pharmaceutical product development process and, thus the future of medical cannabis products, lies in the establishment and application of AOPs. (I initiated the concept of "Adverse Outcome Pathways," or AOPs in Part 2 of this series.) The figure below shows a graphical interpretation of an AOP proposing estrogen receptor antagonism as a (mode of action) MOA for reproductive dysfunction in fish.
An example of an AOP from the AOP-Wiki (“Estrogen receptor antagonism leading to reproductive dysfunction”). Reproduced from https://aopkb.org/aopwiki/index.php/Aop:30.
One can quickly visualize how the information is organized is order to document how a “molecular initiating event” -- in this case, antagonism of the estrogen receptor -- starts a cascade of “key events” up through successive layers of biological organization to produce an “adverse outcome” of decreased spawning and fecundity in individual fish. It is important to note that the diagram above was generated from the existence of published literature and other source of information on the known toxico/pharmacokinetics (how a substance gets into the body and what happens to it in the body) and toxico/pharmacodynamics (the molecular, biochemical, and physiological effects of a substance, or its metabolites, within the body).
In the case of cannabinoids, there is a wealth of older published literature that can be mined to provide the same basic schematic diagram. For example, the structure of cannabidiol was first published in 1963 by Mechoulam and Shvo.
(Raphael Mechoulam is an enormously influential figure in this area of science and is still performing research in this area today, for the Hebrew University of Israel. His work was instrumental in elucidating the existence of the endocannabinoid signaling system.)
In addition to the older literature that lays down basic knowledge of molecular mechanism (see some examples here and here and here), there was the establishment of the understanding of the endocannabinoid system in the 1990s, which fostered a more recent age of high-quality studies (see here and here for some examples of review papers). Some of the areas being explored more recently include:
Angiogenesis (the formation of new blood vessels from pre-existing ones, which can control vascular density in cancerous tumors): some cannabinoids that bind to receptors CB1 and CB2 have been shown to inhibit vascular cell survival and migration (Blazquez et al 2003), suppress production of pro-angiogenesis factors (Blazquez et al 2004) and directly induce vascular endothelial cell death (apoptosis).
- Digestive Disorders: a role for CB2 receptors has emerged from research with patients with inflammatory bowel disease and may represent a "braking system" for gut inflammation and its associated symptoms (as reviewed by Wright et al 2008, also see Kunos and Pacher 2004)
- Neuropathic and chronic pain control: While the evidence base for use of phytocannabinoids to control pain is fairly solid, what is lacking is greater knowledge about the molecular targets of cannabinoid-induced analgesic effects seen in both animal models and limited human clinical trials. More recently, glycine receptors have been investigated as potential potentiators of CB-receptor-mediated processes (as reviewed by Xiong et al 2012)
- Antioxidant properties: Those cannabinoids with a phenol group have mild antioxidant properties to protect neurons from oxidative stress (found in both animal models and in vitro studies) and potential molecular mechanisms are discussed by Borges et al 2013.
The process of establishing AOPs not only provides a framework for organizing the existing information for a chemical’s bioactivity but allows one to pinpoint where the data gaps lie in order to focus later research and/or development of new assays or biomarkers of effect.
Does the binding of the CB1 or CB2 receptor by a phytocannabinoid start a forward cascade that leads to an "adverse" outcome? Or it is the accumulation of binding events at the tissue level that leads to an expression of certain proteins that can be measured and this represents a better (or cheaper?) point to measure to understand bioactivity? It is crucial to note that in the application of this thinking to phytocannabinoid drug production that the OUTCOME in this case is not necessarily ADVERSE, it may be a desired health outcome (like reduction of inflammation through the inhibition of pro-inflammatory cytokines) ... so don't too hung up on the word "adverse" in this sense.
What is now sorely needed to be able to truly harmonize international attempts at understanding the molecular mechanisms of phytocannabinoids is a large-scale AOP project similar to the one run by the OECD. I would liken this to a "Manhattan Project" for establishing networks of AOPs that describe in detail the various pathways under consideration for development of phytocannabinoid medicines. Ideally, information generated would be included in existing relevant databases (i.e. the AOP Knowledge Base) and available in open-access form for all researchers to use. This is already the case with some of the available genomic data.
But this isn't all that's needed to optimize the development of custom blends of cannabinoid drugs that are "fit for purpose" (bespoke) based on biological evidence (i.e. known to perturb a certain pathway at a specific dose leading to a predictable and measurable effect). There is knowledge to be gained from the various genomics initiatives underway as well.
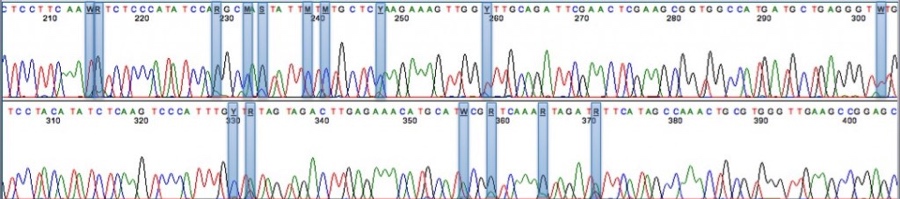
Already, next-generation genomic sequencing technology is being brought to bear to understand the genome of Cannabis sativa and how this can be manipulated to affect strain quality and crop yield (go check out the Medicinal Genomics project!)
This sequencing readout from Medicinal Genomics shows various polymorphisms in the THCA synthase gene, which in part controls genetic variability.
The full genome and transcriptome of Cannabis sativa was published in 2011 and the Cannabis Genome Research Initiative seeks to expand on this work by creating an ultra-high density genetic map, full cultivar history and phylogeny as well as morphologic difference across strains. This information will help optimize strains for production of specific cannabinoids in "cannabis" as well as "hemp" (rich in non-psychoactive CBD but low in psychoactive THC).
Given there are 70-80 phytocannabinoids alone necessitate the use of existing high-throughput modern toxicology approaches. Further given the unknown role that the > 100 terpenoids play along with the known phytocannabinoids only extends that need. Custom combinations of cannabinoids and terpenoids could be studies in the future using knowledge gained through the AOP process in combination with high-throughput screening and molecular and genomic characterization. Current theories regarding the "entourage effect" (great article by another famous researcher, Ethan Russo formerly of GW Pharmaceuticals) could be explored more rapidly given some of the methods discussed above.
In summary, the remarkable advances in technology that have allowed modern toxicology approaches to take root in the pharmaceutical and crop protection industries need to brought to the medical cannabis market. By combining the modern toolbox with the more recent advances in MOA/AOP frameworks, custom “integrated approaches to testing and assessment” (IATA) can be brought to bear that facilitate the collection and organization of data in a way that should save resources and decrease time to the marketplace. It’s time to apply this knowledge to the development of refinement of safe and efficacious medical cannabis & hemp products in a large-scale international research effort: Molecular Mechanisms of Phytocannabinoid Bioactivity.