Poor beleaguered IRIS ... that's the Integrated Risk Information System for those you not up to speed on your EPA acronyms. This EPA program has gotten such a bad rap for so long and it has earnestly tried to change in response to its critics so many times now that it resembles the classic David Byrne lyric (from the equally classic "Life During Wartime"): "I've changed my hairstyle so many times, I don't know what I look like." During this week's public workshop (devoted to detailed discussion of the National Research Council Review of the IRIS Assessment Development Process), yet another change was announced that is worth mentioning. But first a little history.
IRIS was created in 1985 -- and currently housed within the National Center for Environmental Assessment within the Office of Research and Development -- without statutory authority to provide "consensus opinions about the health effects that may result from chronic exposure to various substances in the environment, and to provide these opinions in a database accessible across the Agency." These consensus opinions take the form of a Toxicological Assessment (within which a "toxicity reference values" for subsequent risk assessment is proposed) for an individual substance (or sometimes related group of chemicals), results of which are posted online.
The conclusions of the IRIS program (and the database) have come to be very heavily relied upon as "gold standard" risk assessment inputs by federal, regional and state regulators as well as by many other international experts and students. If you want to know the best reference dose to put in your risk assessment, you go to IRIS. This is what every young risk analyst learns.
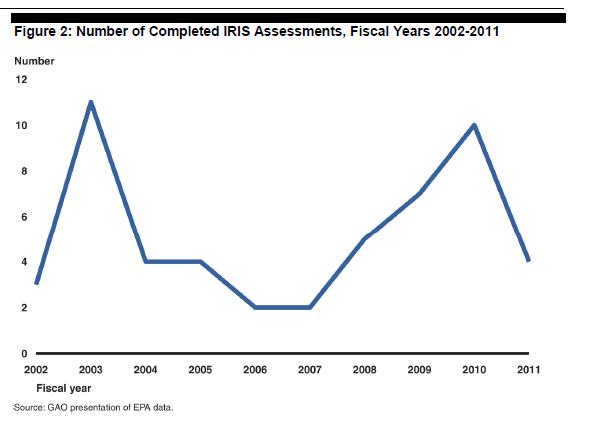
However, the program - since its inception - has basically never been been able to hit its production goals, the assessments it is able to complete take a long time, it no longer serves the needs of other EPA program offices (such as the Pesticides Office, who develop their own tox values for risk asseements) and as a result of all of the above, has been classified as a "high risk" federal program by the GAO which investigates certain wasteful government operations "due to their greater vulnerabilities to fraud, waste, abuse, and mismanagement or the need for transformation to address economy, efficiency, or effectiveness challenges." To quote from the GAO:
"With regard to EPA’s IRIS program, the agency must demonstrate the ability to routinely complete timely, credible IRIS assessments. This will involve developing and achieving, over a sustained period of time, productivity goals for addressing its current backlog of assessments, routinely starting new assessments, and updating existing assessments. EPA must also fully address issues concerning the clarity and transparency of its development and presentation of draft IRIS assessments as well as issues regarding the availability and accuracy of current information to users of IRIS information on the status of IRIS assessments."
IRIS long targeted completion of 20 assessments per year, which it was never able to achieve, As a result of federal GAO scrutiny, NGO scrutiny (see this report from Rena Steinzor), Congressional scrutiny (such as this 2011 hearing or this 2014 hearing), and scrutiny from a seemingly unrelated NRC panel, many changes were put in place by now Director Ken Olden.
None of these changes have resulted in productivity gains to this point and appear to have made the whole public input aspect more complex.
In fact, GAO has been investigating IRIS since 2008:
Chemical Assessments: Challenges Remain with EPA’s Integrated Risk Information System Program. GAO-12-42. Washington, D.C.: December 9, 2011.
High-Risk Series: An Update. GAO-11-278. Washington, D.C.: February 2011.
Chemical Regulation: Observations on Improving the Toxic Substances Control Act. GAO-10-292T. Washington, D.C.: December 2, 2009.
EPA Chemical Assessments: Process Reforms Offer the Potential to Address Key Problems. GAO-09-774T. Washington, D.C.: June 11, 2009.
Chemical Regulation: Options for Enhancing the Effectiveness of the Toxic Substances Control Act. GAO-09-428T. Washington, D.C.: February 26, 2009.
High-Risk Series: An Update. GAO-09-271. Washington, D.C.: January 2009.
Toxic Chemicals: EPA’s New Assessment Process Will Increase Challenges EPA Faces in Evaluating and Regulating Chemicals. GAO-08-743T. Washington, D.C.: April 29, 2008.
Chemical Assessments: Low Productivity and New Interagency Review Process Limit the Usefulness and Credibility of EPA’s Integrated Risk Information System. GAO-08-440. Washington, D.C.: March 7, 2008.
So back to the change announced yesterday.
Per EPA, the ongoing EPA Bimonthly Public Science Meetings "will be supplemented with independent scientific experts identified by the National Academies’ National Research Council ... [and] these independent experts will contribute to the scientific discussion of issues amongst EPA and public commenters." Furthermore, "EPA acknowledges concerns raised by the NRC committee regarding stakeholder participation and has noted that participation amongst stakeholder groups has been uneven during recent IRIS public science meetings. To address the imbalance that has risen from an open registration to participate in IRIS Bimonthly Public Science Meetings, EPA has engaged the NRC to supplement the public discussions with scientific experts identified and assembled by the NRC. These experts, who will be reviewed by the NRC for conflicts of interest and bias, will provide valuable, independent scientific input to these meetings. The involvement of NRC experts will significantly contribute to broadening the range of perspectives represented at our public meetings."
A quick review of the handful of bimonthly public meetings will quickly show one that only industry appeared to have taken the call for public input. The main reason for this is that EPA is taking technical comments on chemicals that industry spends a lot of money and time and effort researching, characterizing and testing. NGOs never have and likely never will sponsor basic research and constructive input from them is often limited to policy comments and reviews of the open literature as mostly published by academics.
NRC was justified in pointing out the imbalance in its May 2014 report, however, EPA is not justified in connecting this the open registration process. One may conclude that EPA is actually interfering in the public input process by "engaging" (i.e. contracting and PAYING) the NRC to select "independent" experts on behalf of the public and to promote a balanced discussion. Furthermore, Olden referred to the new approach as "not a change ... its an enhancement to invite scientists to participate in our bimonthly meetings." It is a change, and its fairly significant.
This author is fully in favor of open, transparent, balanced dialogues concerning regulation and science policy. However, the government shouldn't step in to essentially subsidize independent experts if none rise to the challenge. Perhaps the NGOs that spend the majority of their resources bringing litigation against the federal government should actually assist the government (and themselves in the process!) and spend some of that money more wisely by contracting the academics of their choice to provide technical input at the IRIS bimonthly meetings.
Just because you throw a party and nobody comes ... that doesn't mean you go out to the street and pay somebody to round up some attendees. That might imply you were a bit of an egomaniac. Or desperate. Time will tell for the IRIS program. It clearly must adapt and evolve or die; however, it still does serve an important function to many a risk analyst. Perhaps the lack of consistent progress at IRIS argues for some centralized agency ... or a distributed network of similar duties/mini-programs within the relevant federal agencies.